Flames, essential to numerous industrial processes, vary greatly in their chemical nature and applications. While seemingly simple, the type of flame used can significantly impact the outcome of a process. This article explores the fundamental differences between oxidizing and reducing flames, two key types commonly encountered in various fields.
An oxidizing flame, typically characterized by a bluish color, has more oxygen than necessary for complete combustion, resulting in a hotter and more intense flame. In contrast, a reducing flame, often appearing with a more orange hue, contains insufficient oxygen for complete combustion, which can modify the chemical composition of the material being heated. Understanding these distinctions is crucial for selecting the appropriate flame type for specific applications.
Flame properties such as color and temperature are not merely cosmetic but are indicative of underlying chemical reactions. These characteristics can influence everything from metalwork to culinary techniques, making the knowledge of flame types invaluable for professionals across industries.
Flame Basics
Flame Characteristics
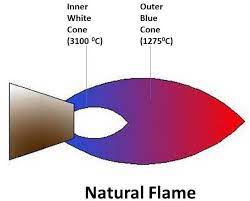
Flames, a fundamental element in many industrial and scientific processes, can be differentiated by color and temperature. These characteristics not only affect their visibility but also their functionality in various applications.
Color Indications
The color of a flame is a direct indicator of its temperature and the nature of the substances being burned. For instance:
- Blue flames are typically hotter, indicating a higher presence of oxygen and efficient combustion.
- Yellow or orange flames suggest incomplete combustion, where oxygen is less available.
The specific color can also reveal the presence of certain elements. Sodium produces a bright yellow flame, whereas copper results in a green color.
Temperature Variations
Temperature differences in flames are crucial for their application. A blue flame, often seen in lab burners and gas stoves, generally reaches temperatures around 1,500°C to 1,700°C. In contrast, a yellow flame might only achieve temperatures up to 1,000°C. These variations are essential for processes requiring precise heat settings.
Oxidation and Reduction
Basic Chemistry
The terms oxidation and reduction refer to chemical reactions involving the transfer of electrons between elements. Oxidation involves the loss of electrons, while reduction involves the gain. These reactions are central to understanding flame behavior.
Role in Combustion
Combustion is an oxidation reaction. In an ideal scenario, the fuel completely reacts with oxygen, releasing heat, light, carbon dioxide, and water. However, variations in this process lead to different flame types, influencing how substances interact with heat.
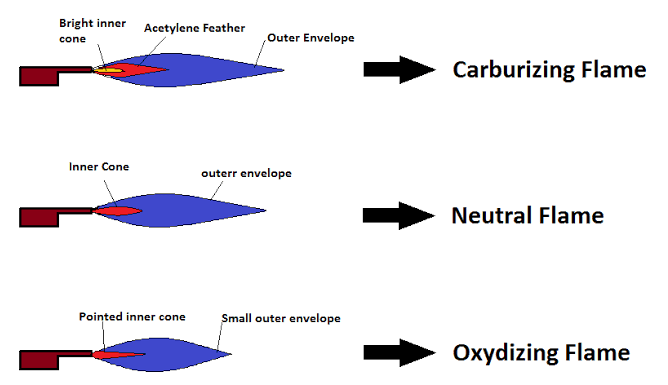
Oxidizing Flame
Defining Features
Appearance and Color
An oxidizing flame is recognized by its sharp, bluish appearance. This color signifies a higher oxygen content, promoting a more intense and hotter flame.
Chemical Properties
Oxidizing flames are characterized by their ability to support the oxidation process. They contain excess oxygen, which can aggressively react with the material being heated, altering its chemical composition and structure.
Applications
Industrial Uses
In industries, oxidizing flames are vital for processes such as:
- Cutting metals: The high temperature and reactive nature of the flame make it ideal for cleanly slicing through metals.
- Glass blowing: Ensures clean and clear glass by preventing soot and other contaminants.
Safety Considerations
Working with oxidizing flames requires strict safety protocols to prevent accidental ignition of surrounding materials. Proper ventilation and flame control are critical to ensure a safe working environment.
Reducing Flame
Core Attributes
Visual and Chemical Traits
A reducing flame, or carburizing flame, typically has a more pronounced orange or reddish appearance. This softer, feathered look indicates lower oxygen levels relative to the fuel, leading to incomplete combustion.
Temperature Specifics
Reducing flames are generally cooler than oxidizing flames. The temperature around the core can be hot, but the outer regions are significantly cooler, which is important for specific applications.
Usage Scenarios
Welding and Metalwork
Reducing flames are essential in scenarios where oxidation of the metal needs to be minimized, such as:
- Welding sensitive metals: Protects the integrity of metals by reducing the risk of oxidation.
- Annealing: Heats metals without altering their properties through excessive oxidation.
Jewelry Making
In jewelry making, reducing flames prevent the oxidation of precious metals, preserving their color and luster during intricate soldering and shaping tasks. This is crucial for achieving high-quality finishes without additional cleaning or polishing stages.
Comparative Analysis
Temperature Comparison
One of the most critical distinctions between an oxidizing flame and a reducing flame is the temperature each can reach. The temperature of a flame directly influences its application and effectiveness in various industrial settings.
- Oxidizing flames can achieve temperatures exceeding 3,000°F (about 1,650°C). This higher temperature is due to the complete combustion facilitated by the excess oxygen, making it suitable for tasks requiring intense heat.
- Reducing flames, on the other hand, typically reach up to 2,500°F (about 1,370°C). The cooler temperature results from incomplete combustion, where less oxygen is available.
Heat Output Differences
The heat output of a flame not only affects the combustion process but also impacts the energy efficiency of a process. Oxidizing flames, with their higher temperatures, can more quickly bring materials to the required temperatures, potentially speeding up operations and enhancing productivity. However, the intense heat can also lead to increased energy consumption.
Reducing flames are often favored in processes where a slower, more controlled heat application is necessary. This can help in reducing thermal shock to materials and allows for finer control over the heating process, which is crucial in delicate operations like glass making or fine metalwork.
Chemical Behavior
Reactions with Metals
The interaction between flames and metals is fundamental in processes such as welding, cutting, and shaping.
- Oxidizing flames may cause certain metals to oxidize rapidly, forming oxides that can weaken the metal’s structure or alter its properties. This is a significant concern when working with metals like aluminum or steel.
- Reducing flames are preferred when working with metals sensitive to oxidation. These flames can help in reducing the oxide layer formation, preserving the metal’s integrity and properties.
Effect on Oxidation States
The oxidation state of a metal can drastically change its characteristics:
- Metals heated in an oxidizing flame often have higher oxidation states, making them harder but more brittle.
- Metals heated in a reducing flame maintain lower oxidation states, which can enhance their malleability and ductility.
Practical Considerations
Choosing the Right Flame
Selecting the correct flame type is essential for achieving optimal results in any thermal process. Here are some factors to consider when choosing between an oxidizing and reducing flame:
- Material sensitivity: Evaluate how the material reacts to different oxygen levels in the flame.
- Desired outcome: Consider whether the process requires higher temperatures or controlled oxidation.
- Efficiency and cost: Factor in the flame’s efficiency and the cost implications of fuel consumption.
Examples from Industry
- In metal cutting, oxidizing flames are preferred for their ability to quickly heat and cut through metals efficiently.
- In artistic glasswork, reducing flames are favored to avoid discoloring or weakening the glass with excessive heat and oxidation.
Safety Tips
Proper handling and safety measures are crucial when working with any type of flame to prevent accidents and ensure a safe working environment.
Handling Procedures
- Check equipment regularly: Ensure that all equipment is in good working condition to avoid leaks and potential fires.
- Proper training: All personnel should be trained in the correct handling of flames, understanding the specific requirements and risks associated with their flame type.
Preventive Measures
- Use protective gear: Always wear appropriate protective gear, such as gloves, goggles, and heat-resistant clothing.
- Maintain a clean workspace: Keep the area free of flammable materials and ensure it is well-ventilated to prevent the buildup of gases.
Frequently Asked Questions
What is an Oxidizing Flame?
An oxidizing flame occurs when the ratio of oxygen to fuel is higher than that needed for stoichiometric combustion, which often results in a shorter, bluer flame. This type of flame is preferred for processes requiring higher temperatures.
What is a Reducing Flame?
A reducing flame, also known as a carburizing flame, has a lower oxygen-to-fuel ratio, leading to incomplete combustion and a longer, orange flame. This flame is crucial for applications where reduced oxidation of the material is necessary.
How Do You Identify a Reducing Flame?
A reducing flame can be identified by its distinct orange or reddish color and a feathered, softer outer flame that indicates lower oxygen levels compared to its fuel-rich core.
Why Choose an Oxidizing Flame?
Oxidizing flames are chosen for their high temperatures and aggressive oxidation characteristics, making them ideal for cutting metals, welding, and other applications where a cleaner and hotter flame is beneficial.
Can Flame Type Affect Material Properties?
Yes, the type of flame used during heat treatment or processing can significantly affect the physical and chemical properties of the material, including hardness, ductility, and corrosion resistance.
Conclusion
The choice between an oxidizing and a reducing flame can drastically affect the efficiency, safety, and quality of industrial and artisan processes. Recognizing the nuances between these flames ensures precise control over the chemical reactions and heat involved, which is essential for achieving desired outcomes in a safe and effective manner.
By understanding the fundamental differences and applications of oxidizing and reducing flames, professionals can optimize their techniques to suit specific tasks, enhancing both product quality and operational safety. This knowledge not only empowers workers but also drives innovation across various industries reliant on thermal processes.