Ozonolysis is a pivotal reaction in organic chemistry, serving as a cornerstone for understanding how alkenes interact with ozone to yield a variety of significant compounds. This chemical reaction, integral to both academic research and industrial applications, splits double bonds through the action of ozone, leading to the formation of different products based on specific conditions and catalysts. The distinction between oxidative and reductive ozonolysis lies in these conditions and the resultant products, which vary dramatically in terms of structure and utility.
Oxidative ozonolysis involves the cleavage of alkenes by ozone and results in the formation of carbonyl compounds, whereas reductive ozonolysis, using a reducing agent like zinc or dimethyl sulfide, typically yields alcohols or ketones. This fundamental difference dictates the applications and methodologies employed in organic syntheses and industrial processes, highlighting the versatility and adaptability of ozonolysis in chemistry.
The process of ozonolysis, whether oxidative or reductive, involves intricate mechanisms that influence the outcome of the reaction. These mechanisms are not just academic curiosities but are crucial for the practical application in synthesizing complex molecules, managing reaction conditions, and optimizing product yields. This understanding is essential for chemists and industry professionals who utilize these reactions to create a wide array of chemical products.
Ozonolysis Basics
What is Ozonolysis?
Ozonolysis is a fundamental reaction in organic chemistry where ozone (O3) interacts with alkenes (carbon-carbon double bonds) to form organic compounds by cleaving these double bonds. This reaction is vital for producing a variety of chemical products and is extensively used in both research and industrial applications. The process involves the addition of ozone to the alkene, resulting in the formation of an unstable ozonide that further reacts to form different products depending on the conditions of the reaction.
Types of Ozonolysis
Ozonolysis can be classified into two main types: oxidative and reductive. Each type follows a different pathway and yields distinct products:
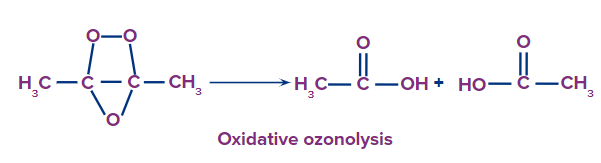
- Oxidative Ozonolysis: This process leads to the formation of carbonyl compounds such as aldehydes, ketones, or carboxylic acids.
- Reductive Ozonolysis: This type uses a reducing agent to prevent full oxidation, typically resulting in the formation of alcohols or ketones.
Oxidative Ozonolysis
Mechanism Explained
The mechanism of oxidative ozonolysis involves several key steps:
- Initial Reaction: Ozone reacts with an alkene to form a molozonide, which is an unstable intermediate.
- Formation of Ozonide: The molozonide rearranges to form a more stable ozonide.
- Decomposition: The ozonide decomposes in the presence of water or in the absence of a reducing agent, leading to carbonyl compounds.
The step-by-step chemical reactions are as follows:
- Alkene + O3 → Molozonide
- Molozonide → Ozonide
- Ozonide + H2O → Carbonyl compounds (e.g., aldehydes, ketones)
Applications
Industrial Uses
In industry, oxidative ozonolysis is used for:
- Manufacturing of Plastics: Precursors to plasticizers and other polymers are often made via oxidative ozonolysis.
- Treatment of Water: Ozone is used to break down pollutants in water through ozonolysis.
Synthetic Applications
In the laboratory, oxidative ozonolysis serves to:
- Synthesize Fine Chemicals: Carbonyl compounds, crucial in pharmaceuticals and agrochemicals, are synthesized through this method.
- Structural Analysis: Determining the structure of complex natural products.
Reductive Ozonolysis
Mechanism Explained
Reductive ozonolysis differs from the oxidative form by incorporating a reducing agent to alter the course of the reaction:
- Initial Reaction: Similar to oxidative, ozone reacts with an alkene to form a molozonide.
- Formation of Ozonide: The molozonide rearranges into an ozonide.
- Reductive Work-up: A reducing agent (e.g., zinc, dimethyl sulfide) is added to convert the ozonide into less oxidized compounds like alcohols or ketones.
The step-by-step chemical reactions are outlined as:
- Alkene + O3 → Molozonide
- Molozonide → Ozonide
- Ozonide + Reducing Agent → Alcohols or Ketones
Applications
Industrial Uses
Reductive ozonolysis finds its importance in:
- Synthesis of Fragrances: Many fragrances are derived from the ketones and alcohols produced.
- Food Industry: Used in the synthesis of flavoring agents and food additives.
Synthetic Applications
In synthetic chemistry, reductive ozonolysis is crucial for:
- Preparation of Drugs: Many pharmaceuticals are manufactured using intermediates obtained from reductive ozonolysis.
- Research and Development: Useful in developing new synthetic pathways for complex molecules.
Comparative Analysis
Key Differences
Reaction Conditions
The conditions under which oxidative and reductive ozonolysis are performed significantly influence their applications and effectiveness. Oxidative ozonolysis generally requires the presence of an oxidizing environment, typically involving an excess of ozone and the absence of a reducing agent. This ensures complete conversion of alkenes to carbonyl compounds. In contrast, reductive ozonolysis requires a reducing agent such as zinc or dimethyl sulfide to prevent the formation of highly oxidized products, instead favoring the production of alcohols or ketones.
Reaction Outcomes
The outcomes of these reactions are distinctly different, reflecting their mechanisms:
- Oxidative Ozonolysis: Produces carbonyl compounds, including aldehydes and ketones, which are often used as key intermediates in chemical synthesis.
- Reductive Ozonolysis: Yields milder oxidation products, primarily alcohols and ketones, which are valuable in less harsh synthetic applications and in industries requiring reduced toxicity.
Advantages
Benefits of Oxidative Method
- Efficiency: Oxidative ozonolysis is highly efficient in breaking down complex alkenes into simpler carbonyl compounds.
- Precision: Offers precise control over the oxidation state of the products, crucial for the synthesis of specific intermediates in pharmaceuticals and material science.
Benefits of Reductive Method
- Safety: Generally safer as it involves less reactive and hazardous conditions due to the presence of reducing agents.
- Selectivity: Provides greater selectivity for desired products, reducing the need for further purification steps and minimizing waste.
Practical Considerations
Safety Measures
Working with ozone requires stringent safety measures due to its highly reactive and potentially hazardous nature. Handling and Safety Tips include:
- Proper Ventilation: Ensure well-ventilated areas to avoid the accumulation of ozone, which can be harmful if inhaled.
- Protective Equipment: Use appropriate personal protective equipment such as gloves, goggles, and face shields.
- Ozone Monitors: Employ ozone monitors in areas where ozonolysis is conducted to detect any leaks or unsafe ozone levels.
Equipment and Materials
Necessary Laboratory Equipment
To conduct ozonolysis safely and effectively, the following equipment is essential:
- Ozone Generator: For the production of ozone from oxygen.
- Reaction Vessels: Specifically designed to handle the reactive nature of ozone.
- Cooling Systems: To maintain the required temperature conditions and stabilize the reaction.
Recent Advances
Innovations in Ozonolysis
Recent technological advancements have significantly enhanced the efficiency and applications of ozonolysis. These innovations include improved catalysts that enhance the reaction rate and selectivity, and new methods that minimize byproduct formation and energy consumption.
Technological Advancements
- Catalyst Development: New catalysts have been developed that allow for lower temperatures and reduced reaction times.
- Automation: Automated systems for the monitoring and control of ozonolysis reactions improve reproducibility and safety.
Research and Development
Continued research in ozonolysis is focusing on making the reaction more environmentally friendly by reducing energy usage and chemical waste. Efforts are also directed towards expanding the utility of ozonolysis in organic synthesis, particularly in the synthesis of complex molecules from simple alkene precursors.
Frequently Asked Questions
What is Ozonolysis?
Ozonolysis is a chemical reaction where ozone decomposes an alkene into smaller organic molecules. It’s a critical method in organic chemistry used to study compound structures and synthesize new materials.
How does Oxidative Ozonolysis work?
In oxidative ozonolysis, ozone reacts with alkenes to form carbonyl compounds such as aldehydes and ketones. This reaction typically does not involve a reducing agent and directly yields the oxidized forms of the organic molecules.
What are the products of Reductive Ozonolysis?
Reductive ozonolysis, unlike its oxidative counterpart, uses a reducing agent to prevent full oxidation and typically results in the formation of alcohols or ketones instead of aldehydes and carboxylic acids.
Why is Reductive Ozonolysis preferred in synthesis?
Reductive ozonolysis is often preferred in synthetic chemistry as it allows for greater control over the products, reducing the formation of potentially harmful or unwanted oxidized byproducts.
What safety measures are needed for Ozonolysis?
Ozonolysis requires stringent safety measures due to the high reactivity of ozone. Proper ventilation, protective clothing, and specific guidelines for handling and disposing of ozone and reaction byproducts are essential.
Conclusion
Ozonolysis remains a technique of profound importance in modern chemistry, bridging the gap between theoretical knowledge and practical application. Its dual variants, oxidative and reductive, offer chemists versatile tools for constructing a broad spectrum of chemical compounds. As research progresses, the nuances of these reactions become clearer, leading to more efficient and safer applications in both laboratory and industrial settings.
The future of ozonolysis looks promising as advances in technology and chemistry continue to enhance its efficiency and safety. The ongoing exploration of this technique will likely unveil new methodologies and applications, solidifying its role in the ever-evolving field of organic chemistry.

