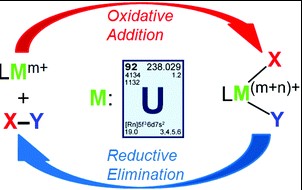
At the heart of modern chemistry lies a duo of pivotal processes: oxidative addition and reductive elimination. These reactions are not just fundamental to understanding chemical transformations; they are the linchpins in the machinery of organic synthesis and catalysis. The nuanced dance between these two reactions can dictate the success of complex chemical syntheses, influencing everything from the production of medicines to the manufacturing of materials.
Oxidative addition involves the increase in oxidation state of a metal as it forms a bond with two additional ligands, expanding its coordination sphere. Reductive elimination, conversely, is a process where a metal complex loses two ligands, decreasing its oxidation state and contracting its coordination sphere. The distinction lies in the direction of electron transfer and bond formation or cleavage, critical for the activation or formation of chemical bonds in catalytic cycles.
The importance of these reactions extends beyond academic curiosity; they are instrumental in the development of sustainable chemical processes and the synthesis of complex molecules. From pharmaceuticals to polymers, understanding the interplay between oxidative addition and reductive elimination enables chemists to design more efficient, selective, and environmentally friendly reactions.
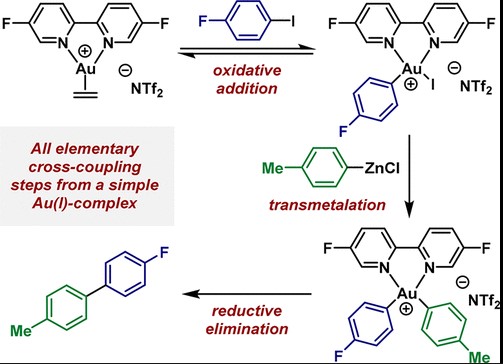
Basic Concepts
Oxidative Addition
Definition: Oxidative addition is a chemical reaction where a metal complex increases its oxidation state by bonding with two additional ligands. This process is essential in organometallic chemistry, expanding the coordination sphere of the metal.
Role in reactions: Oxidative addition is crucial for activating inert chemical bonds, such as C-H, C-C, and many more, making them reactive towards further chemical transformations. This step is vital in catalytic cycles, especially in cross-coupling reactions, where the formation of new bonds is required for synthesis.
Reductive Elimination
Definition: Conversely, reductive elimination is a process where a metal complex decreases its oxidation state by releasing two ligands, which often combine to form a new molecule. This reaction contracts the coordination sphere of the metal.
Reaction significance: Reductive elimination is significant for its ability to form new bonds between ligands while regenerating the catalyst, making it available for another reaction cycle. It’s a cornerstone in the synthesis of complex molecules, playing a pivotal role in finishing synthetic sequences in organic synthesis.
Key Differences
Reaction Mechanism
Oxidative addition mechanism
- Initial State: A metal complex with a low oxidation state.
- Bonding: The metal inserts itself into a bond (e.g., C-H, C-C).
- Final State: Metal’s oxidation state increases; coordination sphere expands.
Reductive elimination mechanism
- Initial State: A metal complex with a higher oxidation state.
- Bond Formation: Two ligands on the metal combine.
- Final State: Metal’s oxidation state decreases; coordination sphere contracts.
Chemical Conditions
Conditions favoring oxidative addition
- Low oxidation state of the metal.
- Presence of a good leaving group.
- Polar solvents, which stabilize the transition state.
Conditions favoring reductive elimination
- High oxidation state of the metal.
- Steric congestion around the metal center.
- Coordination of ligands that promote the desired configuration for elimination.
Outcome
Product of oxidative addition
The outcome is a complex with a higher oxidation state and additional ligands, ready for further reaction steps.
Product of reductive elimination
The outcome is a new molecule formed from the ligands, with the metal complex returning to a lower oxidation state.
Chemical Examples
Oxidative Addition Examples
Example 1: Reaction and analysis
- Palladium(0) catalyzed activation of a bromobenzene results in a Palladium(II) complex. This step is crucial in Suzuki coupling reactions.
Example 2: Reaction and significance
- Iridium(I) complex undergoing oxidative addition with molecular hydrogen (H₂) to form an Iridium(III) hydride. This reaction is fundamental in hydrogenation processes.
Reductive Elimination Examples
Example 1: Detailed examination
- A Platinum(IV) complex undergoing reductive elimination to form ethyl chloride. This step is significant in the synthesis of organochlorides.
Example 2: Industrial relevance
- Nickel(II) complexes in cross-coupling reactions often undergo reductive elimination to form carbon-carbon bonds, a key step in the manufacture of pharmaceuticals.
Role in Catalysis
Oxidative Addition
Impact on catalytic cycles
Oxidative addition is the entry point for many substrates into the catalytic cycle, allowing for the activation of stable bonds.
Applications in synthesis
This reaction is used in the synthesis of complex organic molecules, including drugs and polymers, by enabling the formation of new bonds under mild conditions.
Reductive Elimination
Importance in catalyst regeneration
Reductive elimination regenerates the catalyst, making it available for another cycle, crucial for the sustainability and efficiency of industrial processes.
Examples in organic synthesis
It’s pivotal in the final steps of synthesizing natural products and active pharmaceutical ingredients (APIs), where the formation of precise molecular architectures is required.
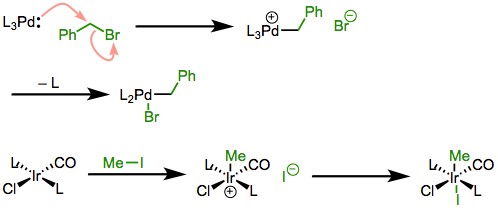
Factors Influencing
Steric Effects
Influence on Oxidative Addition
Steric effects significantly impact the efficiency and selectivity of oxidative addition. Larger, bulkier ligands around the metal center can hinder the approach of substrates, thereby slowing down the reaction. In some cases, sterically hindered ligands are deliberately used to control the reactivity of the metal center, directing the course of the reaction towards a desired pathway or product.
Influence on Reductive Elimination
Similarly, in reductive elimination, steric congestion can either facilitate or impede the reaction. For certain metal complexes, a higher degree of steric bulk can promote reductive elimination by forcing the ligands into proximity, facilitating bond formation. However, excessive steric hindrance can also prevent the proper alignment of ligands for bond formation, thus inhibiting the reaction.
Electronic Effects
Electronic Requirements for Oxidative Addition
The electronic nature of both the metal center and the ligands plays a crucial role in oxidative addition. Metals with low electron density are more susceptible to undergo oxidative addition as they can easily accept electrons from the bond being broken. Ligands that are good electron donors can also facilitate this reaction by increasing the electron density at the metal center, making it a more attractive site for bond insertion.
Electronic Requirements for Reductive Elimination
For reductive elimination, a high electron density at the metal center can hinder the reaction, as the process involves the metal giving up electrons to form a new bond between ligands. Thus, ligands that are poor electron donors or those that can withdraw electron density from the metal are beneficial for facilitating reductive elimination.
Comparative Analysis
Kinetics and Thermodynamics
Kinetic Aspects of Oxidative Addition
Oxidative addition is often the rate-determining step in many catalytic cycles due to the energy required to break the existing bonds in the substrate. The kinetics of this reaction are influenced by the nature of the substrate, the ligand environment around the metal, and the oxidation state of the metal center.
Thermodynamic Stability in Reductive Elimination
Reductive elimination tends to be thermodynamically favorable; however, its occurrence depends on the formation of a stable product. The stability of the product, and thus the driving force for the reaction, is influenced by the bond energies of the new bonds formed between the ligands and the overall energy release in going from a higher to a lower oxidation state of the metal.
Application Scope
Applications in Organic Synthesis
Oxidative addition and reductive elimination are cornerstones in organic synthesis, enabling the construction of complex molecules from simpler precursors. These reactions are key steps in cross-coupling reactions, such as Suzuki, Heck, and Stille couplings, which are pivotal for forming carbon-carbon bonds.
Applications in Pharmaceutical Synthesis
In pharmaceutical synthesis, these reactions enable the formation of complex drug molecules with high efficiency and selectivity. Oxidative addition is used to activate inert bonds, allowing for the introduction of various functional groups, while reductive elimination is critical for forming the final active molecule, often under mild conditions that are compatible with sensitive functional groups.
Advanced Topics
Challenging Substrates
Oxidative Addition with Inert Compounds
Oxidative addition with inert compounds, such as aromatics and alkanes, represents a significant challenge due to their low reactivity. Recent strategies involve the use of highly active catalysts or directing groups to facilitate these reactions, opening new pathways for the synthesis of complex molecules from simple precursors.
Reductive Elimination with Complex Molecules
Performing reductive elimination in the context of complex molecules requires careful consideration of regio- and stereoselectivity to ensure the formation of the desired product. Advances in ligand design have led to more selective catalysts that can guide the outcome of these reactions, even in densely functionalized molecules.
Recent Advances
Latest Research in Oxidative Addition
Recent research in oxidative addition focuses on expanding the scope of reactants, including the activation of challenging bonds like C-H and N-H bonds without the need for directing groups. Innovations in catalyst design are leading to more sustainable and efficient processes, utilizing less toxic metals and operating under milder conditions.
Innovations in Reductive Elimination Techniques
Innovations in reductive elimination are aimed at increasing the efficiency and selectivity of these reactions, particularly in the synthesis of complex molecules. Recent developments include the use of photoredox catalysis to promote reductive elimination under ambient conditions, and the design of novel ligands that enable high selectivity for challenging substrates.
Frequently Asked Questions
What is oxidative addition?
Oxidative addition is a fundamental reaction in organometallic chemistry where a metal complex increases its oxidation state by two units as it forms bonds with two new ligands. This process is crucial for the activation of inert chemical bonds, enabling the formation of new compounds, particularly in catalytic cycles involved in organic synthesis and industrial chemical processes.
How does reductive elimination work?
In reductive elimination, a metal complex undergoes a decrease in its oxidation state by two units, releasing two ligands as a new molecule. This reaction is pivotal in catalysis and organic synthesis, as it often forms the product of a catalytic cycle, enabling the catalyst to be regenerated and used again.
Why are these reactions important in catalysis?
Oxidative addition and reductive elimination are essential steps in many catalytic cycles, particularly in cross-coupling reactions that form carbon-carbon bonds. They allow for the activation of substrates and the formation of products through a series of steps that can be finely tuned for selectivity, efficiency, and sustainability, making them indispensable in the development of new synthetic methodologies.
Can oxidative addition and reductive elimination occur in the same catalytic cycle?
Yes, both oxidative addition and reductive elimination can occur in the same catalytic cycle. They often represent different stages of the cycle, with oxidative addition typically initiating the process by activating substrates and reductive elimination concluding it by forming the final product. This interplay is crucial for the overall efficiency and selectivity of the catalytic process.
Conclusion
Oxidative addition and reductive elimination are more than just reactions; they are the keystones of organometallic chemistry and catalysis, shaping the way chemists think about and approach the synthesis of complex molecules. The ability to manipulate these reactions allows for the exploration of new chemical landscapes, leading to advancements in drug development, materials science, and beyond.
Understanding these processes not only enriches our comprehension of chemical reactivity but also paves the way for future innovations in chemistry. As researchers continue to unravel the intricacies of these reactions, the potential for new and transformative chemical processes grows, highlighting the ever-evolving nature of chemistry and its capacity to address some of society’s most pressing challenges.