Oxidation and reduction are cornerstone concepts in the field of chemistry, pivotal for understanding a myriad of processes from battery operation to cellular respiration. These reactions involve the transfer of electrons between atoms, fundamentally altering their chemical properties and energy states. The potential for an atom to undergo oxidation or reduction is quantified through oxidation potential and reduction potential, respectively, metrics that are essential for predicting the direction and feasibility of chemical reactions.
The difference between oxidation potential and reduction potential lies in their respective measures of an atom’s tendency to lose or gain electrons. Oxidation potential indicates an atom’s eagerness to lose electrons and undergo oxidation, while reduction potential signifies an atom’s propensity to gain electrons and be reduced. These values, often represented under standard conditions, serve as a guide for the likelihood and direction of electron transfer in chemical reactions.
Understanding these potentials not only demystifies the electron transfer process but also highlights their critical role in electrochemical cells, environmental chemistry, and industrial applications. The oxidation potential is inversely related to reduction potential; a higher oxidation potential means a lower reduction potential, and vice versa. This relationship is crucial in determining the outcome of electrochemical reactions, making the distinction between these two potentials fundamental for chemists and industry professionals alike.
Basic Concepts
Oxidation Potential
Definition and Explanation
Oxidation potential is a measure of an atom’s or molecule’s ability to lose electrons and undergo oxidation. In simpler terms, it quantifies how easily an entity can be oxidized. During oxidation, the oxidation state of the atom or molecule increases as it donates electrons. This concept is pivotal in understanding chemical reactions, especially those involving electron transfer.
How It’s Measured
The oxidation potential is typically measured under standard conditions of 1 M concentration, 1 atm pressure, and a temperature of 25°C (298 K). The measurement involves comparing the potential of an electrode under these conditions to a standard hydrogen electrode (SHE), which has an assigned potential of 0 volts. The setup for this measurement includes:
- An electrochemical cell where the substance of interest is one electrode and the SHE is the other.
- A voltmeter to measure the potential difference between the two electrodes.
The resulting voltage gives us the oxidation potential of the substance. A positive value indicates a tendency to lose electrons, while a negative value suggests a lesser propensity for oxidation under standard conditions.
Reduction Potential
Definition and Explanation
Reduction potential, conversely, measures an atom’s or molecule’s ability to gain electrons and be reduced. This potential indicates how readily a substance can accept electrons, reducing its oxidation state. Reduction potentials are fundamental for predicting the direction of electron flow in reactions and for understanding how substances act as oxidizing agents.
How It’s Measured
Like oxidation potentials, reduction potentials are measured under standard conditions against the SHE. The procedure involves:
- Setting up an electrochemical cell with the test substance and SHE.
- Measuring the voltage difference, which directly gives the reduction potential.
A higher reduction potential means a substance is a strong oxidizing agent, eager to gain electrons. These measurements help chemists predict and control the outcomes of redox reactions.
Comparing Potentials
Key Differences
The key differences between oxidation and reduction potentials stem from their definitions:
- Oxidation Potential: Indicates how easily a substance loses electrons.
- Reduction Potential: Shows how readily a substance gains electrons.
The sign of their values also contrasts, with positive oxidation potentials suggesting a tendency to oxidize, whereas positive reduction potentials point to strong oxidizing abilities.
Direction of Electron Flow
The electron flow direction in a redox reaction is from the reducing agent (with a higher oxidation potential) to the oxidizing agent (with a higher reduction potential). This flow is driven by the potential difference between the two reacting substances.
Sign Conventions
- Oxidation Potential: Positive values indicate a greater likelihood of the substance being oxidized.
- Reduction Potential: Positive values signify a strong tendency to reduce, acting as an oxidizing agent.
Interrelation
Oxidation and reduction potentials are intrinsically linked; a high oxidation potential in one substance requires a correspondingly high reduction potential in another for a reaction to occur. The two potentials are essentially different perspectives on the same chemical property – the tendency to transfer electrons.
Example Reactions Illustrating the Interplay
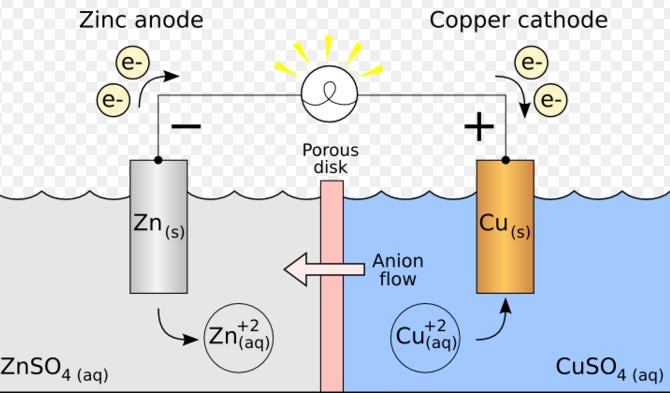
- Zinc and Copper: Zinc has a higher oxidation potential compared to copper, meaning it more readily loses electrons. Copper, with a higher reduction potential, gains these electrons, demonstrating the electron transfer from zinc to copper in a battery.
Electrochemical Series
Explanation of the Series
The electrochemical series is a list of elements ordered by their reduction potentials. It serves as a guide to predict the outcome of redox reactions. Elements at the top have higher reduction potentials, making them strong oxidizing agents, while those at the bottom are weaker and tend to be reducing agents.
Role in Determining Potential Values
This series helps in identifying:
- Which substances can oxidize or reduce each other.
- The direction of electron flow in a reaction.
- Potential reaction spontaneity based on the relative positions of the elements involved.
Calculating Potentials
Standard Conditions
Standard conditions refer to the benchmark settings of 1 M concentration, 1 atm pressure, and 25°C, under which oxidation and reduction potentials are measured. These conditions ensure that potential values are comparable across different substances.
How They Affect Potential Measurements
The environmental factors such as temperature, pressure, and concentration can significantly influence the measured potentials. Standard conditions provide a control point, but real-world conditions may require adjustments in potential values to accurately predict reaction behavior.
Nernst Equation
The Nernst equation allows for the calculation of electrode potentials under non-standard conditions. It adjusts the standard potential values based on the actual reaction conditions like concentration and temperature.
Application in Calculating Potentials
The Nernst equation is applied as follows:
- Identify the standard reduction potential of the reaction.
- Calculate the reaction quotient from the concentrations of reactants and products.
- Apply the Nernst equation to find the actual potential at the given conditions.
Practical Implications
Batteries and Electrochemical Cells
Role of Potentials in Battery Technology
Oxidation and reduction potentials are fundamental in designing and optimizing battery technology. These potentials determine how electrons move between the anode and cathode, which directly affects the voltage and efficiency of the battery. A greater difference between the oxidation potential of the anode material and the reduction potential of the cathode material leads to higher voltage output and more efficient energy storage.
Examples of Common Batteries
- Lead-Acid Batteries: Used in vehicles, these batteries rely on the high oxidation potential of lead and the reduction potential of lead dioxide.
- Lithium-Ion Batteries: Preferred in portable electronics and electric vehicles for their high energy density, leveraging the potentials between lithium cobalt oxide (cathode) and graphite (anode).
Corrosion Processes
How Potentials Predict Corrosion
The corrosion of metals, a natural but undesirable oxidation process, is closely linked to their oxidation potentials. Metals with higher oxidation potentials are more prone to losing electrons and corroding in the presence of oxidizing agents like oxygen and water. This knowledge allows for the prediction and prevention of corrosion in various environments.
Preventive Measures
- Coating: Applying protective coatings to metal surfaces can prevent exposure to corrosive agents.
- Cathodic Protection: By attaching a more easily oxidized metal as a sacrificial anode, the primary metal is protected from oxidation.
- Material Selection: Choosing materials with lower oxidation potentials for specific environments can reduce corrosion risk.
Environmental Impact
Influence on Natural Oxidation and Reduction Processes
Oxidation and reduction potentials play a crucial role in environmental chemistry, influencing natural processes such as the oxidation of organic matter in soils and the reduction of metals in water. These potentials help predict the behavior of pollutants and the feasibility of natural remediation processes.
Examples from Real-life Scenarios
- Water Treatment: The reduction potential of contaminants like heavy metals can inform treatment methods to make water safe for consumption.
- Soil Remediation: Understanding the oxidation potential of pollutants helps in choosing the right strategies for soil cleanup, such as bioremediation.
Challenges and Considerations
Measurement Accuracy
The accuracy of measuring oxidation and reduction potentials is crucial for reliable chemical analysis and industrial applications. Several factors can affect this accuracy, including:
- Electrode Condition: The cleanliness and integrity of the electrodes used in measurements.
- Solution Composition: The presence of impurities or complexing agents in the solution.
- Temperature and Pressure: Deviations from standard conditions can alter potential readings.
How to Ensure Reliable Measurements
Ensuring accurate and reliable measurements involves:
- Regular Calibration: Using standard solutions to calibrate equipment.
- Maintaining Electrodes: Cleaning and replacing electrodes as needed.
- Controlling Conditions: Minimizing deviations from standard conditions during measurements.
Technological Advancements
Recent Developments in Measuring Potentials
Recent advancements in technology have improved the precision and ease of measuring oxidation and reduction potentials. Innovations include:
- High-Precision Instruments: Enhanced sensitivity and stability in potential measurements.
- Digital Data Logging: Automated collection and analysis of data for more accurate and efficient monitoring.
- Portable Devices: The development of compact, field-deployable instruments.
Future Outlook
The future of measuring oxidation and reduction potentials looks promising, with ongoing research focused on:
- Nanotechnology: Using nanomaterials to create more responsive and selective electrodes.
- Wireless Monitoring: Development of wireless sensors for real-time monitoring of potentials in remote or harsh environments.
- Machine Learning: Applying AI to predict potential outcomes based on historical data and trends.
Frequently Asked Questions
What determines oxidation and reduction potential?
Oxidation and reduction potentials are determined by the nature of the chemical species involved and their environmental conditions. Factors such as the element’s electronegativity, the reaction environment (pH, temperature), and the standard state conditions under which these potentials are measured play a significant role. These potentials reflect the inherent energy changes when an atom or molecule gains or loses electrons, influenced heavily by the surrounding chemical landscape.
How do oxidation and reduction potentials impact battery design?
Battery performance hinges on the difference between the oxidation potential of the anode and the reduction potential of the cathode. The greater this difference, the higher the voltage of the battery. Engineers manipulate these potentials through material selection to optimize battery efficiency, capacity, and recharging cycles, thereby tailoring batteries to specific applications from smartphones to electric vehicles.
Can oxidation and reduction potentials predict environmental processes?
Yes, oxidation and reduction potentials can predict environmental processes such as corrosion, metal dissolution, and the behavior of pollutants. For example, the oxidation potential of metallic elements can indicate their susceptibility to corrosion in certain environments, while the reduction potential of contaminants can suggest how they might be naturally degraded or neutralized in water or soil, impacting strategies for environmental remediation and protection.
Conclusion
The exploration of oxidation and reduction potentials provides a window into the electron transfer dynamics that drive chemical reactions. This understanding not only serves as a fundamental aspect of chemical theory but also has practical applications in technology, industry, and environmental management. The distinction between these two types of potential is a key factor in predicting the behavior of chemical systems, from the energy efficiency of batteries to the mechanisms of corrosion and environmental degradation.
By demystifying these concepts, we gain insight into the intricacies of chemical processes and their implications for real-world applications. The knowledge of oxidation and reduction potentials not only enriches our understanding of chemistry but also empowers innovation and problem-solving across a spectrum of scientific and technological fields, highlighting the interconnectedness of science, technology, and the environment.