Chemical reactions are the backbone of both the physical world around us and the biological processes within us. Among these, oxidation and reduction stand out as twin processes that are both opposite and complementary, driving a multitude of chemical reactions. They play a pivotal role in everything from the rusting of iron to the way our bodies process oxygen.
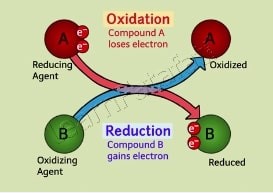
Oxidation and reduction, commonly known as redox reactions, are chemical processes involving the transfer of electrons between two substances. Oxidation occurs when an atom, molecule, or ion loses electrons, while reduction takes place when an atom, molecule, or ion gains electrons. The crux of understanding chemical reactions lies in grasping these two processes, as they often occur simultaneously and are essential for numerous biological and industrial processes.
The significance of oxidation and reduction extends beyond simple electron transfer. These reactions are fundamental to the energy cycles of living organisms, the production of materials, and even the degradation of pollutants. By influencing how substances interact and change, oxidation and reduction shape the chemical landscape of our world, making them fascinating subjects of study within the realm of chemistry.
Basic Concepts
Oxidation Explained
Definition and Process
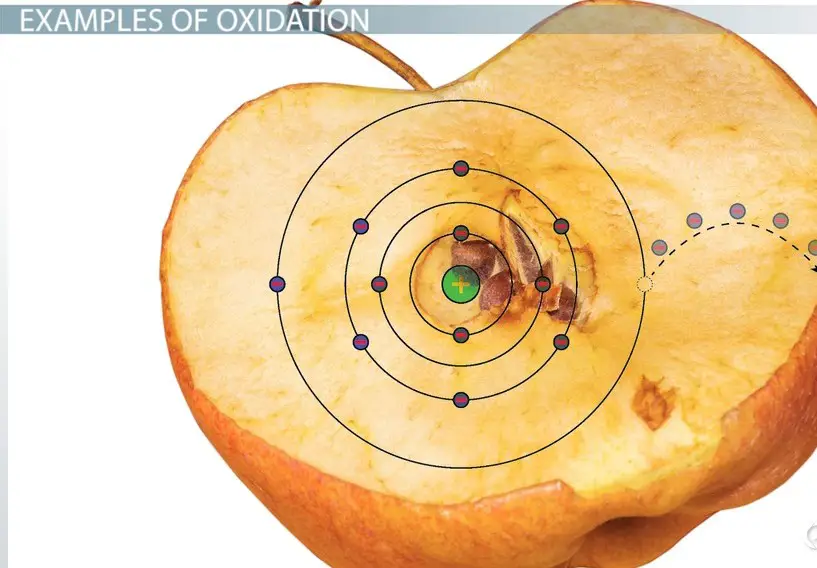
Oxidation is a chemical reaction where an atom, molecule, or ion loses electrons. This process results in an increase in the oxidation state of the substance. A common example of oxidation is the rusting of iron, where iron loses electrons to oxygen.
Historical Context and Origin
The term oxidation was originally used to describe reactions where elements combine with oxygen. Antoine Lavoisier, a French chemist, first used the term in the 18th century. Over time, the definition expanded to include any reaction involving the loss of electrons.
Reduction Explained
Definition and Process
Reduction is the opposite of oxidation. It occurs when an atom, molecule, or ion gains electrons, leading to a decrease in the oxidation state. A simple example is the reduction of iron oxide back to iron in the presence of carbon monoxide during steel making.
Contrast with Oxidation
While oxidation involves the loss of electrons, reduction involves the gain. These two processes are always connected; when one substance is oxidized, another is reduced. This simultaneous occurrence is fundamental to the concept of redox reactions.
Chemical Reactions
Role in Chemical Reactions
Oxidation-reduction (Redox) Reactions
Redox reactions are where oxidation and reduction occur simultaneously. They are essential for various chemical processes, from the metabolic reactions in our bodies to the reactions in batteries that power devices.
Examples in Everyday Chemistry
- Burning fuel: Oxidation of hydrocarbons generates heat and light.
- Breathing: Oxygen is reduced in our bodies to produce water, releasing energy.
- Rusting: Iron oxidizes, forming rust.
Identifying Oxidation and Reduction
Oxidation Numbers
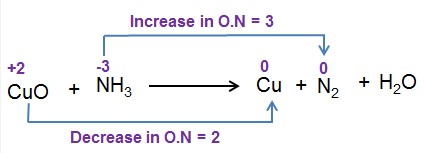
An oxidation number is a value assigned to an element in a compound that represents the number of electrons lost or gained by that element. They help in determining which element is oxidized and which is reduced in a reaction.
Changes in Oxidation State
By comparing the oxidation numbers of elements before and after a reaction, one can identify the oxidized and reduced substances. An increase in oxidation number indicates oxidation, while a decrease indicates reduction.
Molecular Perspective
Electron Transfer
Oxidation as Loss of Electrons
Oxidation at the molecular level involves the transfer of electrons away from a substance. This transfer changes the electrical charge and chemical properties of the substance, often making it more positive.
Reduction as Gain of Electrons
Conversely, reduction involves the acquisition of electrons by a substance. This electron gain typically reduces the substance’s oxidation state, making it more negative or less positive.
Energy Considerations
Exothermic vs Endothermic Reactions
Reactions can either release energy (exothermic) or absorb energy (endothermic). Oxidation and reduction reactions can be either, depending on the substances involved and their chemical bonds.
Role in Energy Production
Redox reactions are crucial for energy production, particularly in biological systems (like cellular respiration) and technology (such as batteries and fuel cells). They facilitate the transfer of energy from chemical bonds to forms that cells or devices can use.
Practical Applications
Industrial Processes
Metal Extraction and Refining
The extraction and refining of metals from their ores often rely on redox reactions. For instance, in the smelting of iron ore, iron oxide is reduced to iron using carbon monoxide. This process not only separates the metal but also refines it, preparing it for industrial use.
Chemical Manufacturing
Many chemicals are produced through redox reactions. The manufacture of sulfuric acid, one of the most important industrial chemicals, involves the oxidation of sulfur dioxide to sulfur trioxide before it is finally reduced to produce the acid. This showcases the role of redox processes in synthesizing compounds critical for various industries.
Biological Systems
Cellular Respiration
Cellular respiration is a complex series of redox reactions that living organisms use to release energy from glucose. Oxygen is reduced to water, and glucose is oxidized to carbon dioxide, demonstrating a vital energy-producing redox process in biological systems.
Photosynthesis
Conversely, photosynthesis involves the reduction of carbon dioxide into glucose using the energy from sunlight. This process is fundamental for life on Earth, converting solar energy into chemical energy through redox reactions.
Environmental Impact
Natural Cycles
Role in the Carbon Cycle
Redox reactions play a significant role in the carbon cycle, which is crucial for regulating Earth’s climate. The decomposition of organic matter, the formation of fossil fuels, and the respiration of living organisms are all redox processes that cycle carbon through the ecosystem.
Impact on Global Climate
These redox reactions, especially those involving carbon dioxide and methane, have a direct impact on the global climate. The balance of these gases in the atmosphere is vital for maintaining the Earth’s temperature, showcasing the environmental significance of redox processes.
Pollution Control
Redox Reactions in Waste Management
Waste management techniques, such as the treatment of wastewater with ozone, rely on redox reactions to break down pollutants. Ozone oxidizes harmful compounds, making them less dangerous and easier to remove.
Technologies for Emission Reduction
In reducing industrial emissions, technologies like catalytic converters use redox reactions to convert harmful gases from vehicle exhaust into less harmful substances, significantly reducing pollution.
Advanced Topics
Catalysis and Speed
Catalysts in Redox Reactions
Catalysts play a crucial role in increasing the speed of redox reactions without being consumed in the process. They work by providing an alternative pathway for the reaction that has a lower activation energy, making the process faster and more efficient.
Impact on Reaction Speed
The presence of a catalyst can dramatically increase the rate of a redox reaction, making many industrial and biological processes feasible. For example, enzymes, which are biological catalysts, speed up the redox reactions in cellular respiration and photosynthesis.
Electrochemistry
Principles of Electrochemical Cells
Electrochemical cells operate on the principles of redox reactions to convert chemical energy into electrical energy. These cells consist of two electrodes immersed in an electrolyte, where oxidation occurs at the anode and reduction at the cathode.
Batteries and Fuel Cells
Batteries and fuel cells are practical applications of electrochemical cells. In batteries, the redox reactions are spontaneous, while in fuel cells, the reaction requires a continuous supply of reactants. Both technologies rely on redox reactions for energy storage and production, highlighting their importance in today’s energy landscape.
Common Questions
Balancing Redox Equations
Basic Strategies and Tips
Balancing redox equations ensures that the number of electrons lost in oxidation equals the number gained in reduction. A simple approach involves:
- Assigning oxidation numbers to all atoms.
- Identifying the atoms oxidized and reduced.
- Using the half-reaction method to balance the electrons transferred.
- Combining the half-reactions and balancing the overall equation.
Real-World Examples
Everyday Examples of Oxidation and Reduction
Redox reactions are not just laboratory phenomena; they occur all around us. For example:
- Breathing: Our bodies use oxygen to oxidize food, releasing energy.
- Rusting: Iron reacts with oxygen and water, forming rust through oxidation.
- Bleaching: Bleach oxidizes stains, removing color and cleaning surfaces.
Frequently Asked Questions
What is Oxidation?
Oxidation is a chemical process in which an atom, molecule, or ion loses electrons. This process is often accompanied by an increase in oxidation state in a reaction. Oxidation plays a vital role in many natural and industrial processes, such as combustion, rusting, and cellular respiration, showcasing its ubiquitous nature in our environment and biological systems.
What is Reduction?
Reduction occurs when an atom, molecule, or ion gains electrons during a chemical reaction, resulting in a decrease in oxidation state. This process is essential for various chemical reactions that sustain life, including the synthesis of DNA and the generation of energy within cells. Understanding reduction is key to grasping the fundamental workings of chemistry and biochemistry.
How Do Oxidation and Reduction Work Together?
Oxidation and reduction reactions, often referred to as redox reactions, work together by transferring electrons from one substance to another. In any redox reaction, while one substance undergoes oxidation by losing electrons, another substance undergoes reduction by gaining those electrons. This interplay is crucial for the flow of energy and the transformation of matter in both technological and biological systems.
Why are Redox Reactions Important?
Redox reactions are crucial for numerous biological processes, such as cellular respiration and photosynthesis, and industrial applications, including the extraction of metals and the production of electrical energy in batteries. Their importance lies in their ability to facilitate the transfer of energy and the transformation of materials, making them foundational to both life on Earth and technological advancement.
Conclusion
Oxidation and reduction are more than just chemical terms; they are processes that underpin the dynamic changes in the world around us. Understanding these reactions offers insights into everything from how metals corrode to how our bodies generate energy. By appreciating the intricacies of electron transfer, we unlock a deeper understanding of the natural and technological phenomena that shape our lives.
In conclusion, the dance between oxidation and reduction is a testament to the complexity and beauty of chemistry. These reactions not only drive the fundamental processes of life but also enable the advancements of modern technology. As we continue to explore and manipulate these processes, we open new avenues for innovation and understanding in science and engineering.