Understanding the difference between orbital diagrams and electron configurations can be an important step in mastering chemistry. This blog will discuss the differences between orbital diagrams and electron configurations, with a focus on how each of them are used to represent electrons in atoms.
We will also explore the advantages and disadvantages of each of these methods of representing electrons. Finally, we will look at how the two representations are related to each other.
Comparing orbital diagrams and electron configurations
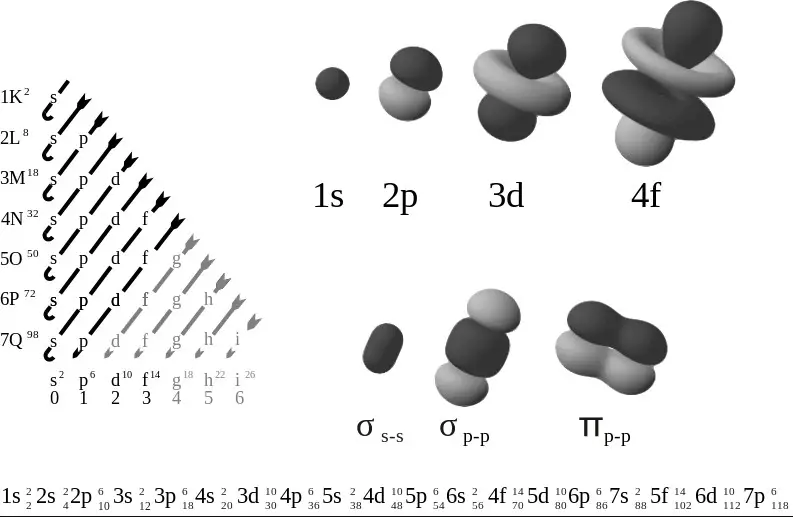
When it comes to understanding the structure of atoms, it is important to understand the difference between orbital diagrams and electron configurations. Orbital diagrams provide a visual representation of the orbital in which electrons are located, whereas electron configurations provide a concise textual representation of the electrons’ location. Orbital diagrams show the number of orbitals, the type of orbital and the number of electrons in each orbital.
Electron configurations provide only the number of electrons in each orbital, with no information about the type of orbital. Orbital diagrams, therefore, provide a more comprehensive view of an atom’s structure.
Detailed explanation of orbital diagrams

Orbital diagrams and electron configurations are often confused, as they both represent electrons in an atom. However, they are quite different.
An orbital diagram is a visual representation of the electrons in an atom, showing the orbitals and the number of electrons in each orbital. On the other hand, an electron configuration is a numerical representation of the electrons in an atom, using numbers and letters to denote energy levels and orbitals. To put it simply, an orbital diagram is like a picture of the electrons in an atom, while an electron configuration is like a written description.
Detailed explanation of electron configurations
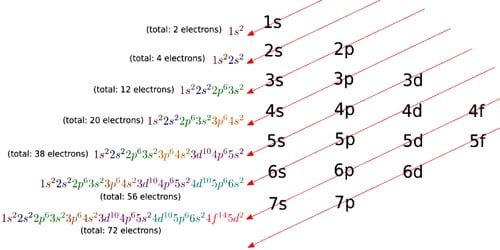
Understanding the difference between orbital diagrams and electron configurations can be a bit tricky. The key difference between the two is that an orbital diagram is a visual representation of the electrons in their orbitals, whereas an electron configuration is a numerical representation. Orbital diagrams are often used to represent the ground state electron configuration of an atom, while electron configurations are used to express the exact placement of the electrons within the orbitals, including the spin of the electrons.
Orbital diagrams are often used to represent the ground state electron configuration of an atom, while electron configurations are used to express the exact placement of the electrons within the orbitals, including the spin of the electrons. By understanding the differences between them, we can get a better understanding of the structure of atoms.
Examples of orbital diagrams and electron configurations
Orbital diagrams and electron configurations are two different ways of representing the arrangement of electrons within an atom. The difference between the two is that an orbital diagram is a visual representation of where electrons are located in an atom, while an electron configuration is a written description of the electron’s location.
An orbital diagram uses arrows to represent the electrons, with each arrow representing one electron. Electron configurations use a series of letters and numbers to indicate the position of the electrons. Both methods are used to accurately describe the arrangement of electrons in an atom, but the orbital diagram is usually easier to interpret and understand.
Benefits of knowing the difference between orbital diagrams and electron configurations
Understanding the difference between orbital diagrams and electron configurations can be a valuable tool in mastering chemistry. An orbital diagram is a visual representation of the orbitals in an atom, while an electron configuration is a description of the arrangement of electrons within the orbitals.
The two concepts are related, but they each provide different information about the atom. By understanding both, you can gain a better understanding of the structure of the atom and how it interacts with other atoms. For example, knowing the electron configuration of an atom can help you determine its reactivity and how likely it is to bond with other atoms.
Similarly, an orbital diagram can be used to show the number and types of electrons present in each orbital, and to predict certain properties of the atom, such as its boiling point. With this knowledge, you can gain a better understanding of the behavior of atoms and how they interact with one another.
Conclusion
In conclusion, the difference between orbital diagrams and electron configurations is that orbital diagrams show the spatial arrangement of the electrons in an atom, while electron configurations show the number of electrons in each orbital. Orbital diagrams are more useful for visualizing the arrangement of electrons, while electron configurations are useful for visualizing the number of electrons in a given atom.
