Isomerism represents a fascinating phenomenon in chemistry where compounds with the same molecular formula exhibit different physical and chemical properties due to variations in the arrangement of their atoms. This concept not only underscores the complexity of chemical compounds but also highlights the intricate relationship between structure and function in the molecular world. Isomers, as these compounds are known, play crucial roles in various scientific fields, from pharmacology to materials science, showcasing the vast implications of atomic configurations.
The difference between optical and geometrical isomerism lies in the spatial arrangement of atoms within a molecule. Optical isomerism occurs when molecules are non-superimposable mirror images of each other, often impacting the way light interacts with them. In contrast, geometrical isomerism involves isomers that differ in their spatial orientation due to restricted rotation around a bond, typically resulting in distinct physical and chemical properties despite having the same connectivity.
Isomerism not only provides a window into the structural nuances of molecules but also offers insights into their functional diversity. Optical isomers, for example, can exhibit vastly different biological activities, a fact that is paramount in drug design and synthesis. Meanwhile, geometrical isomers can alter the physical properties of compounds, influencing their stability, color, and reactivity. Understanding these differences is key to manipulating molecular structures for specific applications, making isomerism a cornerstone of chemical innovation.
Isomerism Basics
Types of Isomerism
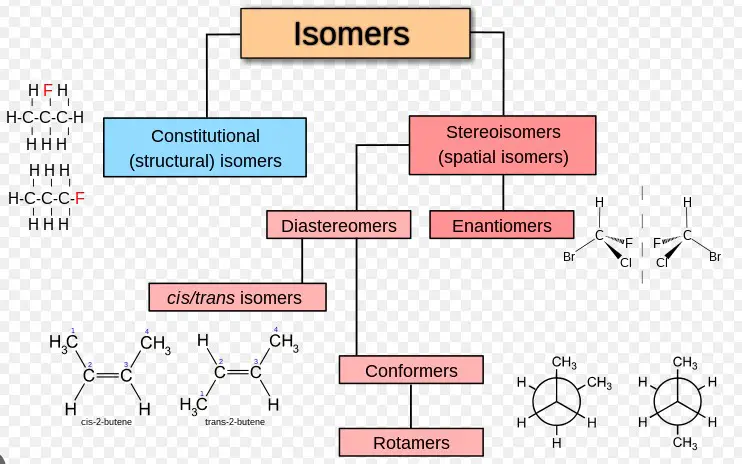
Isomerism is a concept in chemistry where two or more compounds share the same molecular formula but differ in their structure or spatial arrangement of atoms. These variations lead to different physical and chemical properties, making isomerism a cornerstone of organic chemistry and pharmacology.
Structural Isomerism
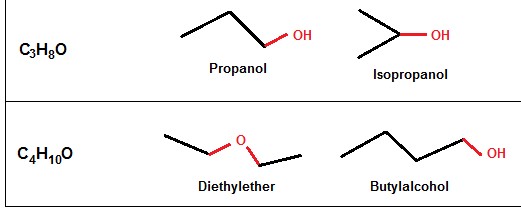
Structural isomerism, or constitutional isomerism, occurs when molecules have the same molecular formula but differ in the connection of atoms within the molecules. The difference in connectivity leads to variations in physical and chemical properties among the isomers.
Examples of structural isomerism include:
- Chain isomerism: Isomers have different carbon chain arrangements.
- Position isomerism: Functional groups are located at different positions on the carbon chain.
- Functional group isomerism: Isomers contain different functional groups.
Stereoisomerism
Stereoisomerism arises when molecules have the same molecular formula and sequence of bonded atoms (constitution) but differ in the 3D orientations of their atoms in space. This form of isomerism is particularly significant because it often results in molecules having drastically different biological activities.
Stereoisomerism Explained
Stereoisomerism is a type of isomerism where molecules have identical molecular formulas and structures but differ in the spatial orientation of atoms. This difference can dramatically affect the chemical behavior and biological activity of the molecules.
Optical and Geometrical Isomerism as Subtypes
- Optical isomerism: Involves isomers that are mirror images of each other but cannot be superimposed. This is primarily due to the presence of chiral centers in the molecules.
- Geometrical isomerism: Occurs due to restricted rotation around a bond, usually a double bond, leading to isomers with different spatial arrangements of their atoms or groups.
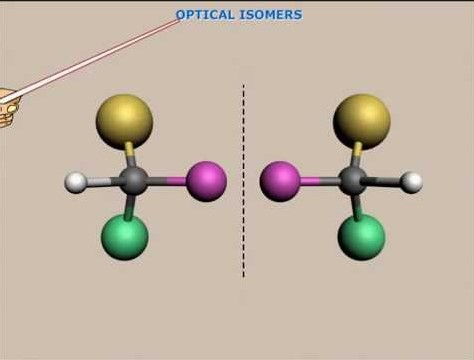
Optical Isomerism
Optical Isomerism Defined
Optical isomerism revolves around the concept of chirality, where molecules are mirror images of each other but cannot be superimposed. These mirror-image molecules, known as enantiomers, exhibit optical activity—the ability to rotate the plane of polarized light.
Fundamental Concepts
- Chirality: Central to optical isomerism, a chiral molecule has a carbon atom bonded to four different groups or atoms, making it asymmetric.
- Optical activity: A measure of how much a substance can rotate the plane of polarized light, a property unique to chiral substances.
Formation and Features
Optical isomers form due to the presence of chiral centers within a molecule. Each chiral center contributes to the potential for optical isomerism, leading to molecules that can exist in two enantiomeric forms.
Characteristics of enantiomers include:
- They have identical physical properties (e.g., boiling point, melting point) but differ in the direction they rotate polarized light (dextrorotary or levorotary).
- Enantiomers can have drastically different biological activities.
Effects in Nature
The biological significance of optical isomerism is profound. Enantiomers can interact differently with biological systems, leading to variations in drug efficacy and metabolism.
Examples in Pharmaceuticals:
- The R-enantiomer of the drug albuterol is effective as a bronchodilator, while the S-enantiomer is less active.
- Thalidomide‘s tragic history underscores the importance of chirality, with one enantiomer being a sedative and the other causing birth defects.
Geometrical Isomerism
Geometrical Isomerism Defined
Geometrical isomerism, also known as cis-trans isomerism, involves isomers that differ in their spatial arrangement around a double bond or within a ring structure where rotation is restricted.
Core Principles
- Cis-trans and E-Z naming systems provide a way to distinguish between isomers based on the relative positions of atoms or groups around the double bond.
Formation and Characteristics
Geometrical isomers arise when there’s restricted rotation around a chemical bond, typically a double bond or within a cyclic structure.
Properties of geometrical isomers:
- Cis isomers have groups on the same side of the double bond, often resulting in higher polarity.
- Trans isomers have groups on opposite sides, which can lead to lower polarity and different physical properties.
Applications and Examples
Geometrical isomerism plays a critical role in chemical and materials science, affecting the physical properties of materials.
Role in Chemical and Materials Science:
- The cis and trans forms of a compound can exhibit different optical and electrical properties, influencing their use in materials science.
Impact on Physical Properties:
- The physical state, melting and boiling points, and solubility of isomers can vary significantly, affecting their practical applications in industrial processes and product formulations.
Differences Highlighted
Comparative Analysis
Key Distinctions in Structure and Formation
The main difference between optical and geometrical isomerism lies in the spatial arrangement of atoms within the molecule. Optical isomerism is characterized by the presence of chiral centers – atoms, typically carbon, bonded to four different groups or atoms. This creates non-superimposable mirror images, or enantiomers. In contrast, geometrical isomerism results from restricted rotation around a bond, leading to cis-trans or E-Z configurations based on the relative positions of substituent groups around a double bond or a ring structure.
Differences in Physical and Chemical Properties
Physical and chemical properties can vary significantly between optical and geometrical isomers. Optical isomers often share similar physical properties (such as boiling point and melting point) but differ in their optical activity, with each enantiomer rotating plane-polarized light in opposite directions. Geometrical isomers, on the other hand, can exhibit notable differences in properties such as solubility, melting point, and chemical reactivity, influenced by the spatial arrangement of the substituent groups.
Implications of Differences
Influence on Biological Activity
The biological activity of molecules can be dramatically affected by isomerism. Optical isomers can interact differently with biological systems, where one enantiomer may be biologically active while the other is inactive or even toxic. This is crucial in drug design, where the desired therapeutic effect must be matched with the correct optical isomer. Geometrical isomers also play a role in biology; for example, the cis configuration of fatty acids results in a different biological function compared to their trans counterparts.
Importance in Industrial Applications
In industrial settings, the specific isomer used can impact product efficiency and safety. In materials science, geometrical isomers can influence the physical properties of polymers and plastics, affecting flexibility, strength, and durability. Optical isomerism is vital in the pharmaceutical industry, where the efficacy and safety of drugs can depend on ensuring the correct enantiomer is produced and administered.
Identifying Isomers
Techniques and Tools
Spectroscopy and Chromatography
To differentiate and identify isomers, scientists employ various analytical techniques:
- Spectroscopy: Techniques like NMR (Nuclear Magnetic Resonance) and IR (Infrared Spectroscopy) provide information on molecular structure, helping identify the presence of chiral centers or double bonds.
- Chromatography: Methods such as GC (Gas Chromatography) and HPLC (High-Performance Liquid Chromatography) can separate isomers based on their physical and chemical properties, allowing for their identification and quantification.
Computational Methods
Computational chemistry tools offer another avenue for identifying isomers. Software can model molecular structures and predict properties, including optical activity and energy states, assisting in the identification of the most stable configurations of molecules.
Challenges and Solutions
Common Challenges in Identification
One of the primary challenges in identifying isomers lies in their often similar physical and chemical properties, making separation and identification difficult. Optical isomers, due to their identical physical properties except for optical activity, require precise techniques to distinguish between them. Geometrical isomers, while different in some physical properties, can still be challenging to separate when the differences are subtle.
Advances in Isomer Separation
Advancements in analytical techniques have greatly improved the ability to identify and separate isomers:
- Chiral chromatography has evolved to efficiently separate enantiomers, using stationary phases that preferentially interact with one enantiomer over the other.
- Two-dimensional NMR spectroscopy provides detailed information on molecular structure, enabling the identification of subtle differences between isomers.
- Mass spectrometry, coupled with advanced separation techniques, can identify isomers based on their mass-to-charge ratio, further elucidating structural differences.
FAQs
What is Isomerism?
Isomerism is a phenomenon where compounds with identical molecular formulas exhibit different physical, chemical, or biological properties due to the arrangement of atoms within the molecules. This unique feature underscores the complexity and versatility of chemical substances, highlighting the significance of molecular structure in determining the characteristics and functionalities of compounds.
How Do Optical Isomers Differ From Each Other?
Optical isomers, or enantiomers, are a pair of molecules that are mirror images of each other but cannot be superimposed. This difference arises from their chiral nature, meaning they have an asymmetry that results in each isomer rotating plane-polarized light in opposite directions. This characteristic significantly affects their interactions with other chiral substances, including biological systems, leading to diverse biological activities.
What Makes Geometrical Isomers Unique?
Geometrical isomers differ in their spatial arrangement due to the restricted rotation around a double bond or a ring structure, leading to variations in the physical and chemical properties of the isomers. This isomerism is often characterized by the terms “cis” and “trans” or “E” and “Z”, which describe the relative positions of the substituent groups attached to the carbon atoms involved in the double bond or ring structure.
Why is Isomerism Important in Pharmaceuticals?
Isomerism plays a crucial role in pharmaceuticals because the biological activity of a drug can significantly vary between different isomers. For instance, one optical isomer of a drug might be therapeutically active, while its mirror image could be inactive or even harmful. Understanding and controlling isomerism is essential for the development of safe and effective medications.
Conclusion
The exploration of optical and geometrical isomerism unravels the profound impact of molecular architecture on the properties and functionalities of compounds. This distinction not only enriches our understanding of chemical diversity but also paves the way for innovations in various scientific and industrial fields. Through the lens of isomerism, researchers and scientists are able to design more effective drugs, develop new materials, and even unravel biological mechanisms, showcasing the boundless potential of chemistry.
As the study of isomerism continues to evolve, its implications extend far beyond the confines of chemistry labs. The ability to manipulate molecular structures opens new horizons in technology, medicine, and environmental science, demonstrating the power of chemistry to solve real-world problems. The journey through the complex landscape of isomerism is a testament to the creativity and ingenuity of scientific inquiry, highlighting the endless possibilities that emerge from understanding the world at the molecular level.