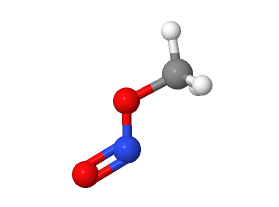
Nitromethane and methyl nitrite, while similar sounding, are distinct chemical compounds with unique properties and applications. Each plays a critical role in various industrial and scientific fields due to their specific characteristics. Understanding the differences between these chemicals is essential for professionals working in chemistry, manufacturing, and environmental science.
Nitromethane is a highly flammable compound commonly used as a fuel additive in racing, as well as a solvent and stabilizer in industrial applications. Methyl nitrite, on the other hand, is a volatile organic compound used primarily in chemical synthesis and pharmaceutical manufacturing. The key difference lies in their molecular structure and resultant physical and chemical properties which dictate their utility and handling requirements.
The significance of these compounds extends beyond their immediate applications. Their impact on environmental safety, health regulations, and technological advancements underscores the importance of accurate knowledge about their characteristics, usage, and safety measures.

Chemical Properties
Nitromethane Overview
Chemical Structure
Nitromethane (CH₃NO₂) consists of a nitro group attached to a methyl group, giving it unique reactivity and properties compared to simpler hydrocarbons. Its molecular structure enables it to act both as a fuel and a solvent, reflecting its dual utility in various industrial contexts.
Key Properties
Nitromethane is known for its high polarity, which makes it an excellent solvent for a wide range of substances. It also exhibits low viscosity and high boiling points, characteristics that are crucial in applications requiring stable and potent solvents. Additionally, its ability to release a significant amount of energy when decomposed thermally or catalytically makes it valuable as a high-energy fuel in motorsports.
Methyl Nitrite Overview
Chemical Structure
Methyl nitrite (CH₃ONO) is composed of a methoxy nitrite group. Unlike nitromethane, its molecular arrangement includes nitrogen connected to an oxygen molecule, imparting different chemical behaviors and risks, particularly in terms of volatility and reactivity.
Key Properties
The key properties of methyl nitrite include its high volatility and reactivity, which are pivotal in synthetic applications. As a gas at room temperature, its uses are often limited by its state, but its ability to act as a methoxylating agent in organic synthesis is highly valued. Additionally, it is sensitive to light and heat, necessitating careful handling and storage conditions.
Production Methods
Nitromethane Synthesis
Industrial Processes
The production of nitromethane typically involves the protonation of propane in a controlled environment. This process requires precise conditions, including specific temperatures and pressures, to ensure high yield and purity:
- Propane is exposed to strong acids.
- Nitration occurs, integrating a nitro group into the propane molecule.
- Refinement processes follow to purify the nitromethane.
Common Uses in Industry
Nitromethane serves a dual role in industry, utilized both as a solvent and as a specialty fuel:
- In the pharmaceutical and chemical manufacturing sectors, its solvent properties help dissolve various organic compounds.
- In motorsports, particularly in drag racing, nitromethane is favored for its ability to significantly increase the engine’s power output without the need for larger volumes.
Methyl Nitrite Synthesis
Industrial Processes
Methyl nitrite is synthesized through the reaction of methanol with nitrous acid, a process that must be carefully managed due to the gas’s volatile nature:
- Methanol is treated with a solution of nitrous acid.
- Reaction conditions are controlled to favor the formation of methyl nitrite.
- The gas is captured and stored under pressure to maintain its stability.
Common Uses in Industry
Primarily, methyl nitrite is used in chemical synthesis:
- It acts as an agent for methoxylation, modifying chemical structures in organic synthesis.
- It is also used in research settings to study reaction mechanisms and pathways due to its reactive nature.
Physical Characteristics
Nitromethane Features
Boiling and Melting Points
Nitromethane has a boiling point of approximately 101 degrees Celsius and a melting point of -29 degrees Celsius. These properties allow it to be used in a variety of environmental conditions, from very cold to relatively high temperatures.
Solubility and Stability
This compound is miscible with most organic solvents but only moderately soluble in water. Its stability is generally good, though it can become unstable and highly explosive when mixed with certain chemicals or exposed to extreme heat.
Methyl Nitrite Features
Boiling and Melting Points
Methyl nitrite has a boiling point around -12 degrees Celsius and is a gas at room temperature, which complicates its handling and storage but is ideal for reactions requiring rapid gas incorporation.
Solubility and Stability
Methyl nitrite is highly reactive and less stable than nitromethane, particularly when exposed to light and heat, which can lead to decomposition or unwanted reactions. Its solubility in water is low, limiting its direct use in aqueous solutions but suitable for organic solvents.

Applications
Uses of Nitromethane
Racing Fuel
Nitromethane is a staple in the world of competitive racing due to its ability to provide a significant increase in power output from engines. When used as a fuel:
- Increases oxygen availability in the combustion process, allowing more fuel to burn and thereby generating more power.
- Enables cars to run at lower air-to-fuel ratios, effectively making smaller engines more powerful and competitive.
- Is primarily used in drag racing, where the short bursts of high power are essential.
Solvent Applications
Beyond the racetrack, nitromethane finds utility in various industrial applications as a potent solvent:
- Cleans heavy-duty machinery and parts, especially where other solvents are ineffective.
- Acts as a medium in chemical reactions where its polarity can facilitate various organic transformations.
- Is used in adhesives and coating formulations to improve application and drying properties.
Uses of Methyl Nitrite
Pharmaceutical Synthesis
In the pharmaceutical industry, methyl nitrite is valued for its role in synthesis processes:
- Facilitates the production of nitroso compounds, which are crucial in making certain pharmaceuticals.
- Acts as a diazotizing agent, a critical step in synthesizing azo dyes and medicinal compounds.
- Used in manufacturing antibiotics and other essential drugs.
Organic Reactions
Methyl nitrite’s reactivity makes it a useful reagent in organic chemistry labs:
- Promotes oxidation reactions that are beneficial in creating complex organic molecules.
- Used in synthesis of cleaning agents and other specialty chemicals that require specific organic reactions.
Health and Safety
Nitromethane Hazards
Toxicity Levels
Nitromethane, while useful, poses significant health risks:
- Highly toxic upon inhalation, causing respiratory issues and potential systemic effects.
- Can be absorbed through the skin, leading to further health complications.
Handling and Storage
Proper practices are critical for safety:
- Storage in cool, well-ventilated areas to prevent accumulation of fumes.
- Use of protective equipment such as gloves, goggles, and respirators during handling.
Methyl Nitrite Hazards
Toxicity Levels
Methyl nitrite is also hazardous, particularly due to its volatile nature:
- Rapidly affects the respiratory system when inhaled.
- Potential for causing methemoglobinemia, a serious condition in which oxygen delivery to tissues is impaired.
Handling and Storage
Methyl nitrite requires stringent safety measures:
- Must be stored under pressure and away from light to prevent decomposition.
- Handling in controlled environments to minimize exposure and prevent health risks.
Environmental Impact
Nitromethane in the Environment
Degradation and Persistence
Nitromethane degrades moderately in the environment but can still pose risks:
- Can persist in water and soil, leading to long-term contamination.
- Biodegradation is slow, and it can accumulate in local environments.
Ecological Effects
The impact on local ecosystems can be significant:
- Toxic to aquatic life, causing potential long-term ecological damage.
- May contribute to ground and water pollution especially if not handled properly.
Methyl Nitrite in the Environment
Degradation and Persistence
Methyl nitrite is highly reactive, which affects its persistence:
- Rapidly decomposes in the presence of sunlight, which limits its lifespan in the environment.
- Volatile nature means it doesn’t accumulate much but can transform into other pollutants.
Ecological Effects
Its transformation products can still impact the environment:
- Forms nitrogen oxides, contributing to air pollution and the formation of ground-level ozone.
- Affects air quality and can have broader impacts on human health and climate.
Regulatory and Legal Status
Nitromethane Regulations
Worldwide Regulations
Nitromethane is regulated globally due to its dual-use nature:
- Listed under various chemical control treaties because of its application in explosives.
- Subject to transport and storage regulations to prevent accidents and misuse.
Specific Country Laws
Countries like the USA and EU have specific guidelines:
- Regulated by the EPA in the USA under the Clean Air Act for its emissions.
- Monitored by the European Chemicals Agency for its environmental and health impacts.
Methyl Nitrite Regulations
Worldwide Regulations
Methyl nitrite also faces stringent oversight:
- Regulated mostly for its impact on air quality.
- Subject to strict handling and storage protocols to prevent occupational and public exposure.
Specific Country Laws
In many regions, additional precautions are mandated:
- Under the US Clean Air Act, it’s monitored for its role in forming ground-level ozone.
- In the EU, regulations focus on worker safety and environmental protection due to its toxicity and reactivity.
Frequently Asked Questions
What is Nitromethane?
Nitromethane is an organic compound with the chemical formula CH₃NO₂. It is used extensively as a fuel in drag racing because of its ability to provide more energy than conventional fuels like gasoline. Additionally, it serves as a solvent and an industrial intermediate in organic synthesis.
How is Methyl Nitrite Produced?
Methyl nitrite is produced through the reaction of methanol and nitrite salts. This process is typically carried out under controlled conditions in chemical plants to ensure safety and efficiency. Methyl nitrite is primarily used in chemical synthesis and as a reagent in various organic reactions.
Are Nitromethane and Methyl Nitrite Dangerous?
Both compounds have significant safety concerns. Nitromethane is highly flammable and explosive under certain conditions, while methyl nitrite is a potent oxidizer and can be toxic if inhaled. Proper handling, storage, and safety protocols are crucial to prevent accidents and ensure safe usage.
Can Nitromethane and Methyl Nitrite Affect the Environment?
Yes, both chemicals can impact the environment if not managed properly. Nitromethane can contribute to air and water pollution due to its volatile nature and combustion byproducts. Methyl nitrite also poses risks as it can lead to the formation of ground-level ozone, a key air pollutant.
Conclusion
The distinctions between nitromethane and methyl nitrite are not only fundamental but pivotal for their respective fields of use. Recognizing and understanding these differences is crucial for the safe and effective application of each compound. Moreover, their impact on environmental and regulatory frameworks cannot be overlooked, necessitating ongoing research and adaptation of usage practices.
As the demand for specialized chemicals grows in various sectors, the role of nitromethane and methyl nitrite is likely to expand and evolve. This underscores the importance of continual learning and adaptation by professionals in related industries to harness their properties effectively while mitigating associated risks.