Nitrogen compounds play a pivotal role in various facets of our lives, from agricultural practices to environmental management. Among these compounds, NH3-N and NH4-N stand out as key players, each with distinct characteristics and implications. In this article, we unravel the complexities surrounding NH3-N and NH4-N, shedding light on their differences and significance in different contexts.
In a nutshell, NH3-N refers to ammonia nitrogen, primarily existing in the form of ammonia gas or dissolved ammonia in aqueous solutions. On the other hand, NH4-N denotes ammonium nitrogen, predominantly found as the ammonium ion in solution. Understanding the disparity between these two forms of nitrogen is crucial for addressing environmental concerns, optimizing industrial processes, and ensuring public health and safety.
As we delve deeper into the realm of NH3-N and NH4-N, we embark on a journey to explore their chemical compositions, environmental impacts, analytical methods for detection, and much more. Join us as we uncover the intricacies of these nitrogen compounds, providing valuable insights for professionals, researchers, and enthusiasts alike.
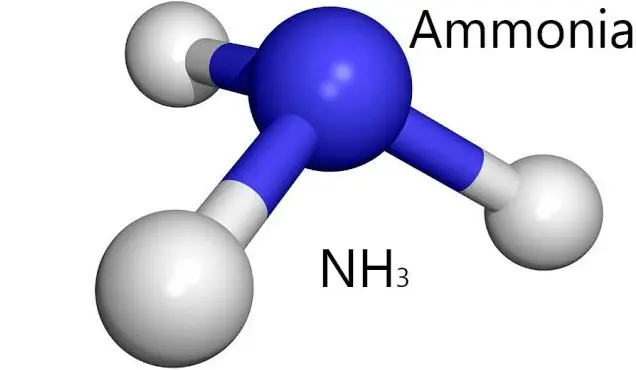
What is NH3-N?
NH3-N, or ammonia nitrogen, is a crucial compound in various industries and environmental contexts. Understanding its fundamental properties and applications is essential for professionals across different fields. Let’s delve into the intricacies of NH3-N to grasp its significance.
Explanation of NH3-N
NH3-N refers to the presence of nitrogen in the form of ammonia (NH3) in aqueous solutions or as gaseous ammonia. Ammonia is a pungent, colorless gas with a distinct odor, often soluble in water to form ammonium hydroxide. In solution, ammonia exists in equilibrium between the gaseous and ionized forms, making it versatile in various processes.
Chemical Composition and Properties
The chemical composition of NH3-N comprises one atom of nitrogen bonded to three hydrogen atoms. This simple molecular structure belies its complex behavior and interactions in different environments. Ammonia is basic in nature, capable of reacting with acids to form salts, known as ammonium compounds.
Sources of NH3-N in the Environment
NH3-N emanates from diverse sources, including agricultural activities, industrial processes, and natural decomposition. Livestock farming, fertilizer application, and animal waste contribute significantly to ammonia emissions. Industrial sectors such as chemical manufacturing, wastewater treatment, and petroleum refining also release ammonia into the environment.
Importance in Various Industries and Applications
NH3-N plays vital roles in numerous industries, ranging from agriculture to manufacturing. In agriculture, ammonia-based fertilizers enrich soil fertility and promote crop growth. In the chemical industry, ammonia serves as a precursor for producing various nitrogenous compounds, including nitric acid, urea, and ammonium nitrate. Moreover, ammonia’s versatility extends to refrigeration, cleaning agents, and pharmaceuticals, highlighting its ubiquitous presence in daily life.
Key Differences Between NH3-N and NH4-N
Understanding the distinct characteristics of NH3-N and NH4-N is imperative for comprehending their roles in environmental and industrial settings. Let’s explore the key differences between these two nitrogen compounds and their implications.
Solubility and Form
NH3-N and NH4-N exhibit contrasting solubility and forms in aqueous solutions, influencing their behavior and interactions with the environment.
NH3-N: Gaseous vs. Dissolved Form
Ammonia exists primarily in two forms: gaseous and dissolved. In aqueous solutions, ammonia can dissolve to form ammonium hydroxide (NH4OH), contributing to the alkalinity of the solution. The equilibrium between ammonia gas and ammonium ions governs its concentration and availability in aquatic environments.
NH4-N: Existence as Ammonium Ion in Solution
In contrast, NH4-N predominantly exists as the ammonium (NH4+) ion in solution. Ammonium ions result from the protonation of ammonia in water, forming a stable compound with a positive charge. This ionic form of nitrogen is readily assimilated by plants and microorganisms, contributing to nutrient cycling and ecosystem dynamics.
Impact on Water Quality and Aquatic Ecosystems
The presence of NH3-N and NH4-N in water significantly influences water quality and the health of aquatic ecosystems.
NH3-N: Toxicity to Aquatic Organisms
Excessive levels of NH3-N can pose serious threats to aquatic organisms, particularly fish and invertebrates. Ammonia toxicity occurs when un-ionized ammonia (NH3) enters the bloodstream of aquatic organisms, disrupting physiological processes and causing cellular damage. Therefore, monitoring and controlling ammonia levels are critical for maintaining aquatic biodiversity.
NH4-N: Utilization by Plants and Microorganisms
NH4-N, on the other hand, serves as a vital nutrient source for plants and microorganisms in aquatic ecosystems. Ammonium ions are readily absorbed by plant roots and serve as a primary source of nitrogen for growth and development. Microorganisms play a crucial role in the nitrification process, converting NH4-N into nitrate (NO3-), which is further utilized by plants and algae.
Role in Nitrogen Cycle and Nutrient Cycling
NH3-N and NH4-N are integral components of the nitrogen cycle, facilitating nutrient cycling and ecosystem stability. The conversion of organic nitrogen into ammonia by decomposition processes initiates the nitrogen cycle, wherein NH4-N undergoes assimilation, nitrification, and denitrification processes, ultimately returning nitrogen to the atmosphere. This cyclic process maintains nutrient balance and sustains biological productivity in aquatic environments.
Environmental Implications and Significance
The environmental implications of NH3-N and NH4-N extend beyond aquatic ecosystems, affecting air quality, regulatory standards, and water management practices.
NH3-N: Air Pollution and Odor Issues
NH3-N emissions contribute to atmospheric pollution, forming particulate matter and reactive nitrogen compounds. Agricultural activities, livestock farming, and industrial processes are major sources of ammonia emissions, leading to air quality deterioration and odor issues in surrounding areas. Inhalation of ammonia gas can adversely affect human health, causing respiratory problems and exacerbating existing conditions.
NH4-N: Nutrient Enrichment and Eutrophication
Excessive NH4-N levels in water bodies can lead to nutrient enrichment and eutrophication, triggering algal blooms and oxygen depletion. The influx of nutrients, including ammonium ions, promotes the rapid growth of algae and aquatic plants, altering ecosystem dynamics and jeopardizing water quality. Eutrophication poses challenges for ecosystem management and necessitates strategies for nutrient control and mitigation.
Regulatory Considerations and Water Quality Management
Regulatory agencies and governing bodies enforce standards and guidelines for NH3-N and NH4-N levels in water bodies, aiming to protect public health and preserve aquatic ecosystems. Monitoring programs, compliance measures, and technological innovations play key roles in ensuring water quality and sustainability. Integrated approaches to water management, including source control, treatment technologies, and public awareness, are essential for mitigating nitrogen pollution and promoting environmental stewardship.
Analytical Methods for Detection and Measurement
Accurate detection and measurement of NH3-N and NH4-N are essential for environmental monitoring, regulatory compliance, and risk assessment initiatives.
NH3-N: Direct Methods vs. Indirect Methods
Analyzing NH3-N concentrations requires robust methods capable of detecting low levels of ammonia in aqueous solutions and ambient air. Direct methods, such as colorimetric assays and gas sensors, offer rapid and real-time measurements of ammonia concentrations. Indirect methods, including titration and spectrophotometry, provide precise quantification of ammonia levels through chemical reactions and spectral analysis.
NH4-N: Common Techniques for Quantification
Similarly, NH4-N quantification employs various techniques, each with distinct advantages and applications. The Nesslerization method, based on colorimetric reactions with Nessler’s reagent, is widely used for rapid determination of ammonium concentrations. Ion-selective electrodes and chromatographic methods offer high sensitivity and specificity for NH4-N analysis, suitable for research and environmental monitoring purposes.
Frequently Asked Questions
What are the main sources of NH3-N in the environment?
NH3-N primarily originates from agricultural activities, including livestock farming, fertilizer application, and organic matter decomposition. Industrial processes such as wastewater treatment plants and chemical manufacturing also contribute to NH3-N emissions into the environment.
How does NH4-N affect water quality?
NH4-N, in its ammonium ion form, can lead to nutrient enrichment in aquatic ecosystems, potentially triggering eutrophication and harmful algal blooms. Moreover, high levels of NH4-N can pose risks to aquatic organisms and compromise water quality, impacting both human and environmental health.
What analytical methods are commonly used to measure NH3-N and NH4-N?
Analyzing NH3-N typically involves techniques such as colorimetry, titration, and spectrophotometry. NH4-N measurement commonly utilizes methods like the Nesslerization method, ion-selective electrodes, and chromatography. Each method offers varying levels of accuracy, precision, and sensitivity, tailored to specific analytical requirements.
Conclusion
In conclusion, the distinction between NH3-N and NH4-N is not merely semantic but holds profound implications for various sectors. From mitigating air pollution to safeguarding water resources, these nitrogen compounds demand our attention and understanding. By comprehending their differences and harnessing their potential, we can pave the way for sustainable development and environmental stewardship. Join us in our commitment to unraveling the mysteries of NH3-N and NH4-N, shaping a brighter and greener future for generations to come.