Sodium borohydride (NaBH4) and Lithium aluminium hydride (LiAlH4) are two pivotal reagents in the realm of organic synthesis, each serving distinct roles depending on their chemical properties and reactivity. In the laboratory, the choice between these two can significantly influence the outcome of chemical reactions, often determining the efficiency and success of a synthesis.
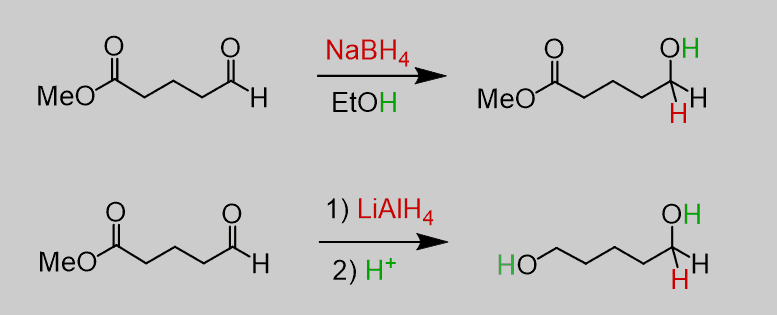
NaBH4 is generally milder and more selective in reducing aldehydes and ketones to alcohols, whereas LiAlH4 is more potent, capable of reducing not only aldehydes and ketones but also esters, carboxylic acids, and amides to alcohols. This fundamental difference in reactivity profiles makes them indispensable tools in organic chemistry, each suited for specific types of chemical transformations.
Both reagents exhibit unique behavior under various conditions, and their correct usage is pivotal in achieving desired results in synthesis. This involves a nuanced understanding of their properties, such as reaction mechanisms, solvent compatibilities, and environmental and safety considerations, which are crucial for efficient application in both academic and industrial settings.
Reagent Profiles
NaBH4 Basics
Structure and Properties
Sodium borohydride (NaBH4) is a white, crystalline powder known for its role as a reducing agent in organic chemistry. Its molecular structure consists of a sodium ion bonded to a borohydride group, which is responsible for its chemical properties. The borohydride anion, BH4-, is the active site of reduction, offering a set of lone electrons that make it highly effective for certain types of chemical reactions.
Key properties include:
- Stability under normal conditions, allowing it to be stored without special requirements.
- Reactivity with water or alcohols, typically in controlled conditions to prevent rapid decomposition.
Common Uses in Chemistry
NaBH4 is renowned for its selective reduction of aldehydes, ketones, and imines to alcohols without affecting other functional groups like esters or carboxylic acids. This selectivity makes it a preferred choice in:
- Synthesizing complex molecules in pharmaceuticals, where gentle and precise reduction is necessary.
- Material science, for the production of specialty materials where specific functional groups are preserved.
LiAlH4 Essentials
Structural Insights
Lithium aluminium hydride (LiAlH4) is another potent reducing agent, comprising a lithium ion and an aluminium hydride group. This structure imparts a high degree of reactivity, especially against a wide range of organic functional groups.
Notable structural features include:
- Reactivity that is significantly higher than that of NaBH4, capable of reducing more robust bonds.
- Sensitivity to moisture, requiring careful handling and storage in dry conditions.
Typical Applications
LiAlH4 finds extensive use in reducing esters, carboxylic acids, and amides to alcohols. Its ability to break stronger bonds makes it essential in:
- Advanced organic syntheses, such as in the preparation of complex organic molecules in drug manufacturing.
- Hydrolysis reactions in polymer and materials engineering to create specific polymer structures.
Key Differences
Reactivity Comparison
Overview of Reactivity Levels
Comparing NaBH4 and LiAlH4, the latter is significantly more reactive. This heightened reactivity allows LiAlH4 to engage in reactions that NaBH4 cannot facilitate, such as the reduction of esters and carboxylic acids.
Factors Influencing Reactivity
- Electronegativity of the metal ion: Li in LiAlH4 is less electronegative than Na in NaBH4, influencing the electron density at the hydride ion and enhancing its reducing power.
- Ionic radius also plays a role, with lithium’s smaller size facilitating a more compact and reactive structure.
Solvent Compatibility
Solvent Choices for NaBH4
NaBH4 is typically used in:
- Alcohols like methanol and ethanol, which moderately control its reactivity.
- Aqueous solutions for specific reductions where enhanced solubility is required.
Solvent Preferences for LiAlH4
Due to its reactive nature, LiAlH4 is generally used in:
- Aprotic solvents such as diethyl ether and tetrahydrofuran (THF), which prevent unwanted side reactions with solvent molecules.
Reaction Mechanisms
NaBH4 Mechanism
Step-by-Step Breakdown
- Formation of a complex between the borohydride ion and the carbonyl group of the substrate.
- Transfer of a hydride ion from borohydride to the carbonyl carbon.
- Protonation of the alkoxide ion to form the final alcohol.
Types of Reactions Facilitated
- Reduction of aldehydes and ketones: Primarily to primary and secondary alcohols.
- Selective reduction in multistep syntheses where other functional groups must remain unaffected.
LiAlH4 Mechanism
Detailed Process Description
LiAlH4 reacts by initially forming an alkoxide complex with the substrate, followed by the transfer of hydride ions. This step is more vigorous compared to NaBH4, enabling the reduction of more resistant functional groups.
Reaction Versatility
LiAlH4 is utilized for:
- Comprehensive reductions, converting carboxylic acids, esters, and amides to alcohols.
- Amide to amine conversion, which is a unique capability not typically seen with NaBH4.

Practical Applications
Uses in Industry
Industrial Synthesis Examples
Sodium borohydride (NaBH4) and lithium aluminium hydride (LiAlH4) are fundamental in various industrial applications due to their reducing capabilities. For instance, NaBH4 is used extensively in the manufacture of bulk pharmaceuticals and fine chemicals, where it selectively reduces aldehydes and ketones. This specificity is crucial in maintaining the integrity of sensitive compounds during synthesis.
LiAlH4, due to its stronger reducing power, is employed in scenarios that require more robust reactions, such as the reduction of esters to alcohols. This is often seen in the synthesis of complex organic compounds, where complete reduction is necessary.
Role in Pharmaceuticals
In the pharmaceutical industry, these reagents play critical roles in drug design and development. NaBH4 is often used to reduce imines to amines, a key step in the synthesis of many active pharmaceutical ingredients (APIs). LiAlH4’s ability to reduce almost all carbonyl-related functional groups makes it indispensable for creating a broad range of APIs, ensuring the efficacy and safety of therapeutic drugs.
Laboratory Applications
Common Lab Procedures
In academic and research laboratories, both reagents are pivotal for teaching and research purposes. Typical procedures include:
- Reduction experiments in organic chemistry classes using NaBH4 to demonstrate the selectivity and mechanism of reducing agents.
- Synthesis projects where LiAlH4 is used to fully reduce complex molecules, often in graduate-level research.
Safety and Handling Tips
Safety is paramount when handling these potent chemicals. Important tips include:
- Always handle in a fume hood: Vapors from these reactions can be hazardous.
- Use proper personal protective equipment (PPE): Gloves, goggles, and lab coats are essential.
- Avoid moisture: Both reagents react violently with water, thus should be handled under dry conditions.
Selecting the Right Reagent
Criteria for Selection
Comparison of Costs
Cost-effectiveness is a key criterion. NaBH4 is generally less expensive and more available than LiAlH4, making it a more cost-effective choice for reductions that do not require the extra strength of LiAlH4.
Availability and Storage
NaBH4 is stable and easy to store, making it more accessible for routine use. LiAlH4, being highly reactive, requires stringent storage conditions to prevent moisture ingress and degradation, influencing its availability and handling requirements.
Decision Factors
Project-Specific Considerations
The choice between NaBH4 and LiAlH4 often depends on the specific requirements of the chemical reaction. For example, if a reaction involves sensitive functional groups that must be preserved, NaBH4 might be the better option. Conversely, for more comprehensive reductions, LiAlH4 would be necessary.
Environmental and Safety Impacts
Both reagents require careful consideration of their environmental and safety impacts. NaBH4, being milder, has a relatively lower risk profile compared to LiAlH4. The use of LiAlH4 needs stringent safety measures due to its aggressive reactivity and potential for hazardous byproducts.
Frequently Asked Questions
What is NaBH4 used for?
NaBH4 is primarily used in the reduction of aldehydes and ketones to alcohols. It is favored for its mildness and selectivity, making it ideal for sensitive reductions where excessive reaction conditions might lead to undesirable byproducts.
How does LiAlH4 react with esters?
LiAlH4 reacts vigorously with esters, reducing them to primary alcohols. It breaks the ester linkage, unlike NaBH4, which is not strong enough to affect esters. This makes LiAlH4 a more versatile reagent in scenarios where complete reduction is required.
Can NaBH4 reduce carboxylic acids?
No, NaBH4 is not effective in reducing carboxylic acids to alcohols. For such reductions, stronger reagents like LiAlH4 are necessary, which can fully reduce carboxylic acids to primary alcohols through more vigorous reaction conditions.
Is LiAlH4 safe to use in all solvents?
LiAlH4 is highly reactive, especially with protic solvents like water and alcohols, which can lead to violent reactions. Typically, it is used in dry, aprotic solvents such as ethers (e.g., diethyl ether, THF) to control and safely conduct its reactions.
Conclusion
In summary, both NaBH4 and LiAlH4 are essential reagents in organic synthesis, each with distinct characteristics that make them suitable for specific chemical reactions. Choosing the right reagent depends on the nature of the substrate and the desired outcome, highlighting the importance of understanding their chemical properties and reaction conditions.
As we continue to explore the capabilities and applications of these reagents, their role in advancing the field of chemistry remains undeniable. Whether in academic research or industrial applications, the informed use of NaBH4 and LiAlH4 is crucial for achieving precise and efficient synthetic transformations.