Solubility and miscibility are foundational concepts in chemistry that play critical roles in various scientific, environmental, and industrial processes. While both terms describe the mixing behavior of substances, they apply to different scenarios and follow distinct rules. The understanding of these concepts not only furthers scientific knowledge but also aids in practical applications ranging from medication formulation to environmental protection.
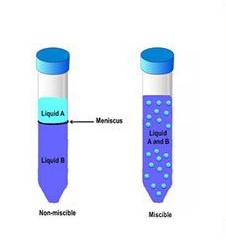
The difference between miscible and soluble lies in the phase and extent of mixing. Miscible substances are those that can mix in any proportion to form a homogeneous solution, typically referring to liquids. Soluble substances, on the other hand, dissolve in a solvent to a certain limit, which can involve solids, liquids, or gases. This distinction is crucial for predicting and controlling the behavior of mixtures in various settings.
These phenomena are influenced by molecular interactions, including polarity, hydrogen bonding, and Van der Waals forces, along with environmental conditions like temperature and pressure. Understanding these interactions allows for the precise use of substances in creating products, from pharmaceuticals to everyday items, and in managing the environmental impact of chemical spills and pollutants.
Basic Concepts
Solubility Explained
Definition
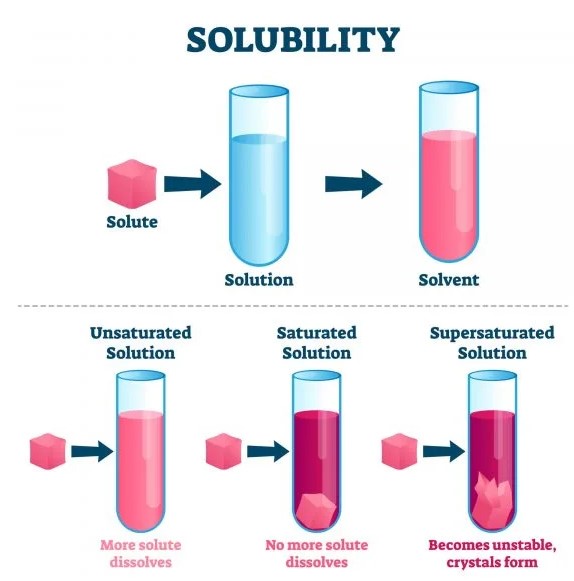
Solubility refers to the ability of a substance, known as the solute, to dissolve in a solvent to form a homogeneous mixture or solution. The extent to which a solute can dissolve in a solvent varies widely and is quantified as its solubility.
Factors Influencing Solubility
Several factors affect how well a solute can dissolve in a solvent. These include:
- Temperature: Generally, solubility increases with temperature for solids and liquids, while gases tend to be more soluble at lower temperatures.
- Pressure: For gases, solubility in liquids increases with pressure (Henry’s Law). This effect is less pronounced for solids and liquids.
- Nature of Solute and Solvent: The chemical nature and polarity of both the solute and solvent play a crucial role. “Like dissolves like” is a helpful principle; polar solutes are
more soluble in polar solvents, while non-polar solutes dissolve better in non-polar solvents.
Miscibility Defined
Definition
Miscibility is a term used to describe the ability of two liquids to mix in any proportion without forming two separate phases. When liquids are miscible, they will form a single homogeneous solution regardless of the amount mixed.
Conditions for Miscibility
The primary condition for miscibility is the compatibility of intermolecular forces between the two liquids. Factors influencing miscibility include:
- Polarity: Similar to solubility, polar liquids are generally miscible with other polar liquids, and the same applies to non-polar liquids.
- Temperature: The miscibility of some liquid pairs can change with temperature.
- Molecular Size and Structure: Differences in size and structure can affect miscibility.
Key Differences
Phase Interaction
Solids in Liquids vs. Liquids in Liquids
The process of a solid dissolving in a liquid involves the solute breaking down into its individual components and interacting with the solvent. In contrast, miscibility refers exclusively to the mixing of liquids, where each retains its molecular integrity but distributes uniformly throughout the other.
Concentration Limits
Saturation Points vs. Complete Mixing at All Proportions
Solubility is often limited by a saturation point, beyond which no more solute can dissolve in the solvent, leading to excess solute. Miscibility, on the other hand, allows for complete mixing of liquids in all proportions without reaching a saturation point.
Temperature and Pressure
Effects on Solubility vs. Miscibility
Temperature and pressure significantly affect solubility, especially for gases in liquids, where an increase in pressure increases solubility, and an increase in temperature generally increases the solubility of solids and liquids but decreases it for gases. Miscibility between two liquids is less influenced by pressure but can be affected by temperature changes.
Molecular Interactions
Polarity and Hydrogen Bonding in Solubility
Polarity and hydrogen bonding are critical for solubility. Solutes with polar molecules or those capable of hydrogen bonding tend to dissolve well in solvents with similar characteristics due to the strong intermolecular forces.
Van der Waals Forces in Miscibility
Miscibility is often governed by Van der Waals forces, especially in non-polar liquids. These weaker forces allow non-polar liquids to mix easily, as there is less energy required to overcome the molecular interactions between the molecules.
Practical Applications
Industrial Uses
Solvents in Manufacturing
Solvents play a crucial role in manufacturing, from pharmaceuticals to paints. Their ability to dissolve other substances makes them invaluable in creating solutions, suspensions, and emulsions needed in various products.
Alloys in Material Science
Although not a direct application of solubility or miscibility, the concept of mixing substances to achieve desired properties is similar. Alloys are mixtures of metals that are melted and mixed together to enhance strength, durability, or resistance to corrosion.
Everyday Examples
Medicines and Solubility
The solubility of active pharmaceutical ingredients in solvents determines the effectiveness of medication delivery methods. Soluble compounds can be easily absorbed by the body, making the medication more effective.
Alcohol and Water Miscibility
Alcohol and water are miscible in all proportions, which is why alcoholic beverages have a uniform composition, regardless of the amount of alcohol they contain. This miscibility is due to the similar polarity of alcohol and water molecules.
Environmental Impact
Solubility in Water Pollution
The solubility of pollutants in water determines their impact on water quality. Soluble pollutants can disperse more easily, affecting larger areas and making them more challenging to remove.
Miscibility in Oil Spills
Oil, being generally non-polar, does not mix well with polar water, leading to significant environmental damage in oil spills. The lack of miscibility causes oil to float on water surfaces, hindering the cleanup process and affecting marine life.
Testing and Measurement
Solubility Tests
Methods for Determining Solubility
To accurately measure solubility, scientists use several techniques. These methods help in understanding how substances interact with solvents, crucial for pharmaceutical, chemical, and environmental applications.
- Gravimetric Analysis: This method involves dissolving a known amount of solute in a solvent, allowing it to reach saturation, then removing and weighing the undissolved solute. The difference in weight indicates how much solute has dissolved.
- Spectrophotometry: A technique that measures how much light a solution absorbs. Since absorption is related to concentration, this method can determine the solubility by analyzing the solution’s color intensity.
- Conductivity Tests: For ionic solutes, measuring the electrical conductivity of a solution can indicate solubility. An increase in ions enhances conductivity, allowing scientists to gauge the amount of solute dissolved.
Miscibility Observations
Techniques for Assessing Miscibility
Miscibility can be observed through simple qualitative tests, unlike the quantitative measurements required for solubility. These observations rely on visual cues and behavior patterns when two liquids are mixed.
- Visual Inspection: Simply mixing two liquids and observing any separation into layers can immediately indicate miscibility. A homogeneous mixture means the liquids are miscible.
- Turbidity Measurement: Mixing two liquids and observing any cloudiness or turbidity can help assess miscibility. A clear solution indicates miscibility, while cloudiness may suggest partial miscibility or immiscibility.
- Temperature Variation: Some liquids may only be miscible at certain temperatures. Gradually changing the temperature and observing the mixture can provide insights into their miscibility range.
Factors Affecting Both
Temperature
Influence on Both Phenomena
Temperature plays a critical role in both solubility and miscibility. For most solid solutes, an increase in temperature leads to increased solubility in liquids. This is because higher temperatures provide more energy to overcome the forces keeping solute molecules together. However, for gases, solubility in liquids decreases with an increase in temperature, as gases have more energy to escape the solvent. In the case of miscibility, temperature changes can either enhance or reduce the mixing of liquids, depending on the nature of the substances involved.
Pressure
Impact on Solubility and Miscibility
While pressure significantly affects the solubility of gases in liquids—increasing with higher pressure due to the gas molecules being “pushed” into the solution—it has a minimal direct impact on the solubility of solids and liquids. In contrast, pressure changes have little to no effect on the miscibility of liquids, as liquid volumes are relatively incompressible compared to gases.
Chemical Structure
Role of Molecular Size and Shape
The chemical structure, including molecular size and shape, influences both solubility and miscibility. Large, bulky molecules might have difficulty dissolving or mixing due to steric hindrance, where the physical space the molecules occupy prevents interaction with the solvent or another liquid. Similarly, the shape of molecules can affect how well they can pack together or interact with solvent molecules, influencing both solubility and miscibility.
Misconceptions Clarified
Soluble vs. Dissolvable
Clarifying Common Misunderstandings
A common confusion arises between the terms soluble and dissolvable. While both refer to the ability of a substance to mix with a solvent, soluble is often used in a more specific context to describe the extent to which a substance can dissolve. Dissolvable is more general, indicating that a substance can dissolve in a solvent without specifying how much or to what extent. Understanding this distinction is crucial for accurate communication in scientific and industrial contexts.
Miscibility as Complete Solubility
Differentiating Between the Two
Another common misconception is equating miscibility with complete solubility. While miscible liquids can mix in any proportion to form a homogeneous solution, solubility usually has limits. Soluble substances can only dissolve up to a certain point, known as the saturation point, beyond which additional solute will not dissolve. This key difference highlights the unique nature of miscibility as an unlimited blending of liquids, distinct from the constrained dissolution process in solubility.
Frequently Asked Questions
What Determines a Substance’s Solubility?
A substance’s solubility is determined by its chemical structure, the solvent’s properties, temperature, and pressure. Molecular interactions such as polarity and hydrogen bonding play significant roles. For instance, polar substances tend to be soluble in polar solvents, while non-polar substances are more likely to dissolve in non-polar solvents.
Can Miscibility Be Influenced by Temperature?
Yes, temperature can significantly influence miscibility. For example, an increase in temperature can enhance the miscibility of two liquids by providing the energy needed to overcome intermolecular forces. This is why some liquids that do not mix at lower temperatures may become miscible at higher temperatures.
How Is Solubility Measured?
Solubility is typically measured as the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure, expressed in units such as grams per liter (g/L). Various methods, including gravimetric analysis and spectrophotometry, can be used to determine solubility.
Why Is Understanding Miscibility Important in Environmental Science?
Understanding miscibility is vital in environmental science for managing the spread and impact of pollutants, especially in water bodies. For instance, miscible pollutants can disperse more widely and may require different remediation strategies compared to non-miscible substances.
Conclusion
Grasping the distinction between miscible and soluble substances is essential for navigating the complexities of chemistry and its application in the real world. This understanding underpins much of the work in pharmaceuticals, environmental science, and industrial processes, where precise control over substance interactions leads to innovation and environmental protection.
The insights into how and why substances mix—or don’t—equip professionals and enthusiasts alike with the knowledge to predict outcomes, solve problems, and innovate within their respective fields. As we continue to explore and manipulate the molecular world, the principles of solubility and miscibility remain central to our ability to create, protect, and sustain our environment and health.