Greenhouse gases play a pivotal role in shaping the climate of our planet, among which methane and fluorinated gases are significant yet distinctly different contributors. While both types of gases are known for their impact on the Earth’s atmosphere, their sources, uses, and effects vary greatly. This introduction aims to set the stage for a deeper exploration into their differences and implications for environmental policy.
Methane is a simple hydrocarbon, a potent greenhouse gas that occurs both naturally and from human activities, significantly influencing climate change. Fluorinated gases, although less common, have a much higher global warming potential, making them critical in discussions about climate impact. Their differences lay in chemical properties, lifespans in the atmosphere, and their industrial applications.
Focusing on their environmental roles, methane’s relatively shorter atmospheric lifetime contrasts with some fluorinated gases that can persist for thousands of years. This durability makes fluorinated gases particularly challenging to manage in the context of global warming and ozone layer depletion, highlighting the need for targeted environmental policies and innovative reduction strategies.
Methane Basics
Chemical Composition
Methane (CH4) is the simplest hydrocarbon, consisting of one carbon atom bonded to four hydrogen atoms. This structure makes methane a highly effective heat-trapper in the Earth’s atmosphere. It is both naturally occurring and produced through human activities, contributing significantly to the greenhouse gas effect.
Common Sources
Methane is emitted from a variety of sources that can be broadly categorized into natural and anthropogenic (human-made). Natural sources include wetlands, where bacteria produce methane as they decompose organic material without oxygen. Other natural sources are termites, oceans, and hydrates.
Human-related sources include:
- Agriculture, especially from the digestive processes of ruminant animals like cows and sheep.
- Landfills, where organic waste decomposes anaerobically.
- Energy sector, particularly through the extraction and burning of fossil fuels such as coal, oil, and natural gas.
Role in Global Warming
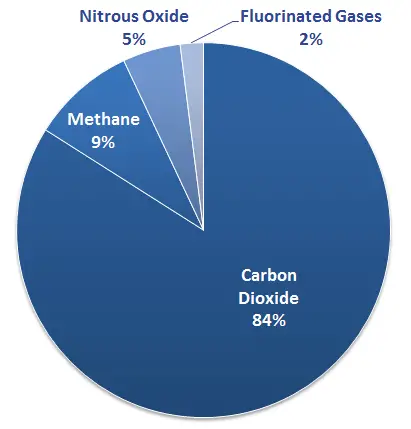
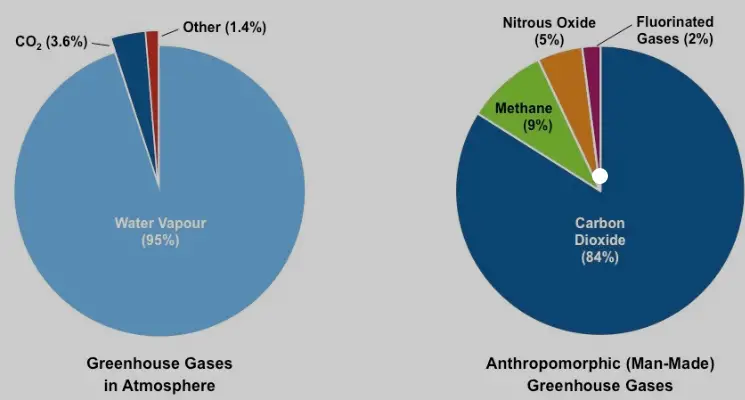
Methane is a potent greenhouse gas, with a global warming potential 28-36 times greater than carbon dioxide over 100 years. This means it can trap significantly more heat in the atmosphere than carbon dioxide over the same period. Although methane only stays in the atmosphere for about 12 years, its immediate impact on climate change is much higher, making it a crucial target for climate change mitigation efforts.
Fluorinated Gases Overview
Definition and Types
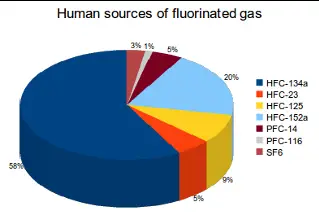
Fluorinated gases, often referred to as F-gases, are a group of synthetic compounds that contain fluorine. They include hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), sulfur hexafluoride (SF6), and nitrogen trifluoride (NF3). These gases are known for their extreme stability, which makes them useful in various industrial applications but also poses significant challenges for the environment.
Common Applications
Fluorinated gases are used in a range of applications due to their non-flammable and high-performance properties. Common uses include:
- Refrigeration and air conditioning systems.
- Aerosol propellants in medical inhalers and other spray products.
- Solvents for cleaning in the semiconductor and electronics manufacturing industries.
- Fire suppression systems and insulating foams.
Contribution to Climate Change
Fluorinated gases have very high global warming potentials, ranging from hundreds to thousands of times greater than carbon dioxide, depending on the gas and its formula. Their long atmospheric lifetimes allow them to remain in the atmosphere for decades to centuries, contributing significantly to long-term climate change.
Chemical Properties
Molecular Structures
Methane has a tetrahedral structure with a carbon atom at the center. This geometry allows for even distribution of electrons, which contributes to its stability under normal conditions. In contrast, fluorinated gases often have complex structures that incorporate fluorine atoms, known for their strong electronegativity, enhancing the stability and reactivity of these gases.
Reactivity and Stability
Methane reacts with oxygen to form carbon dioxide and water, a reaction that is both a source of energy and a contributor to atmospheric carbon levels. Fluorinated gases, however, are generally more stable due to the strong bonds between carbon and fluorine, resisting degradation in the lower atmosphere and contributing to their longevity and impact in the upper atmosphere.
Environmental Impact
Atmospheric Lifetimes
Methane’s relatively short atmospheric lifetime of about 12 years contrasts sharply with that of some fluorinated gases, which can persist for thousands of years. This difference significantly influences their respective roles and management strategies in climate policy.
Global Warming Potentials
The global warming potential (GWP) of fluorinated gases is notably high. For example, SF6 has a GWP of 23,500 over 100 years. This means it is 23,500 times more effective at trapping heat than carbon dioxide over the same period, highlighting the critical need for careful management and regulation of these gases.
Effects on Ozone Layer
While methane does not directly deplete the ozone layer, some fluorinated gases, particularly CFCs and HCFCs (which are being phased out), have been known to contribute to ozone depletion. Their replacements, such as HFCs, do not deplete the ozone layer but are potent greenhouse gases that need to be managed to prevent further climatic impacts.
Industrial Uses
Methane Applications
Methane serves as a fundamental resource in numerous industrial sectors, primarily due to its abundance and energy content. Major applications of methane include:
- Energy Production: Methane is a primary component of natural gas, used extensively to generate electricity and heat through combustion processes.
- Chemical Synthesis: It acts as a raw material in the production of chemicals such as methanol and hydrogen, which are further utilized in creating products ranging from plastics to pharmaceuticals.
- Fuel: Compressed natural gas (CNG) and liquefied natural gas (LNG) are both derived from methane, offering alternatives to traditional petroleum-based fuels for vehicles.
Uses of Fluorinated Gases
Fluorinated gases find their utility in various advanced industrial applications, highlighting their critical role despite environmental concerns. Common uses include:
- Electronics Manufacturing: Utilized as etching gases to make circuits and components in the semiconductor industry.
- Refrigerants: Key in cooling technologies, these gases are used in air conditioning systems in cars and buildings.
- Medical Applications: Employed in imaging devices and as an inhalation agent in some anesthetic applications due to their inert characteristics.
Regulatory Frameworks
Global Regulations
The global response to the challenge posed by greenhouse gases like methane and fluorinated gases has led to the development of several international agreements, such as:
- Kyoto Protocol: Targets industrialized nations to reduce their emission levels and includes mechanisms that govern emissions of fluorinated gases.
- Paris Agreement: While not specifically targeting methane or fluorinated gases, it encourages countries to frame national policies to minimize overall greenhouse gas emissions.
National Policies
Countries adopt specific national strategies to regulate and manage the emissions of these gases:
- United States: Implements regulations through the Environmental Protection Agency (EPA), which include mandatory reporting of greenhouse gas emissions from large sources and best practices for leakage reduction in the oil and gas sector.
- European Union: The EU has stringent regulations on fluorinated gases, aiming to cut their use by two-thirds by 2030 through the F-gas Regulation, which includes restrictions on their use and related products.
Reduction Strategies
Technological Solutions
Innovations in technology play a pivotal role in mitigating the impact of methane and fluorinated gases:
- Methane Capture and Utilization: Technologies to capture methane from landfills and livestock, converting it into energy, thereby reducing emissions.
- Refrigerant Reclamation and Recycling: Systems designed to recover and recycle fluorinated gases from air conditioning and refrigeration equipment.
Policy and Regulation Impact
Effective policies and regulations are critical in shaping the reduction strategies for these gases. They influence industrial practices and promote the adoption of green technologies:
- Incentives for Low-Emission Technologies: Financial incentives such as tax credits or subsidies to support the adoption of emission-reducing technologies.
- Regulatory Mandates: Requirements for leak detection and repair in gas transmission, mandatory performance standards for new appliances, and phasedown schedules for the most potent fluorinated gases.
Future Trends
Innovations in Reduction
Research and development continue to drive forward new methods to reduce the environmental footprint of these gases:
- Advanced Membranes and Absorbents for Gas Separation: Novel materials that can efficiently separate methane from other gases or capture fluorinated gases before they are emitted into the atmosphere.
- Biological Methane Conversion: Emerging techniques that utilize bacteria or other microorganisms to convert methane into less harmful substances or usable products.
Predicted Changes in Usage
Anticipated future changes in the industrial use of methane and fluorinated gases include:
- Decrease in Methane for Energy: As renewable energy sources become more prevalent, the reliance on methane for power generation is expected to decrease.
- Transition Away from Fluorinated Gases in Refrigeration: With ongoing regulatory pressures and environmental concerns, industries are likely to shift towards more sustainable alternatives like hydrofluoroolefins (HFOs) and natural refrigerants.
Frequently Asked Questions
What are greenhouse gases?
Greenhouse gases are compounds in the Earth’s atmosphere capable of absorbing infrared radiation, trapping heat in the atmosphere, and contributing to the greenhouse effect. Methane and fluorinated gases are two examples, each with unique properties and impacts on the climate.
How do methane and fluorinated gases differ?
Methane, a hydrocarbon, is primarily released through natural processes and human activities such as agriculture and fossil fuel extraction. Fluorinated gases, synthetic compounds used in various industrial applications, are powerful greenhouse gases with longer atmospheric lifetimes and higher global warming potentials.
What are the main sources of methane?
The main sources of methane include natural sources like wetlands and human-induced sources such as livestock farming, landfills, and the burning of fossil fuels. Methane is also a significant byproduct of oil and natural gas development.
Why are fluorinated gases significant in climate discussions?
Despite their lower concentrations, fluorinated gases are significant in climate discussions due to their high global warming potentials. They can trap much more heat per molecule than carbon dioxide, making them potent contributors to long-term climate change.
Conclusion
Methane and fluorinated gases, while both contributing to the greenhouse effect, present different challenges and require distinct approaches for mitigation. Understanding these gases in detail is crucial for developing effective environmental policies and technologies aimed at reducing their impact. This knowledge not only informs global regulatory efforts but also guides industries in adopting more sustainable practices.
As we advance, the role of scientific research and technological innovation becomes increasingly vital in managing the emissions of these gases. Continued efforts to improve emission detection and reduction strategies will be key to addressing the complex challenges posed by methane and fluorinated gases in our quest to mitigate climate change.