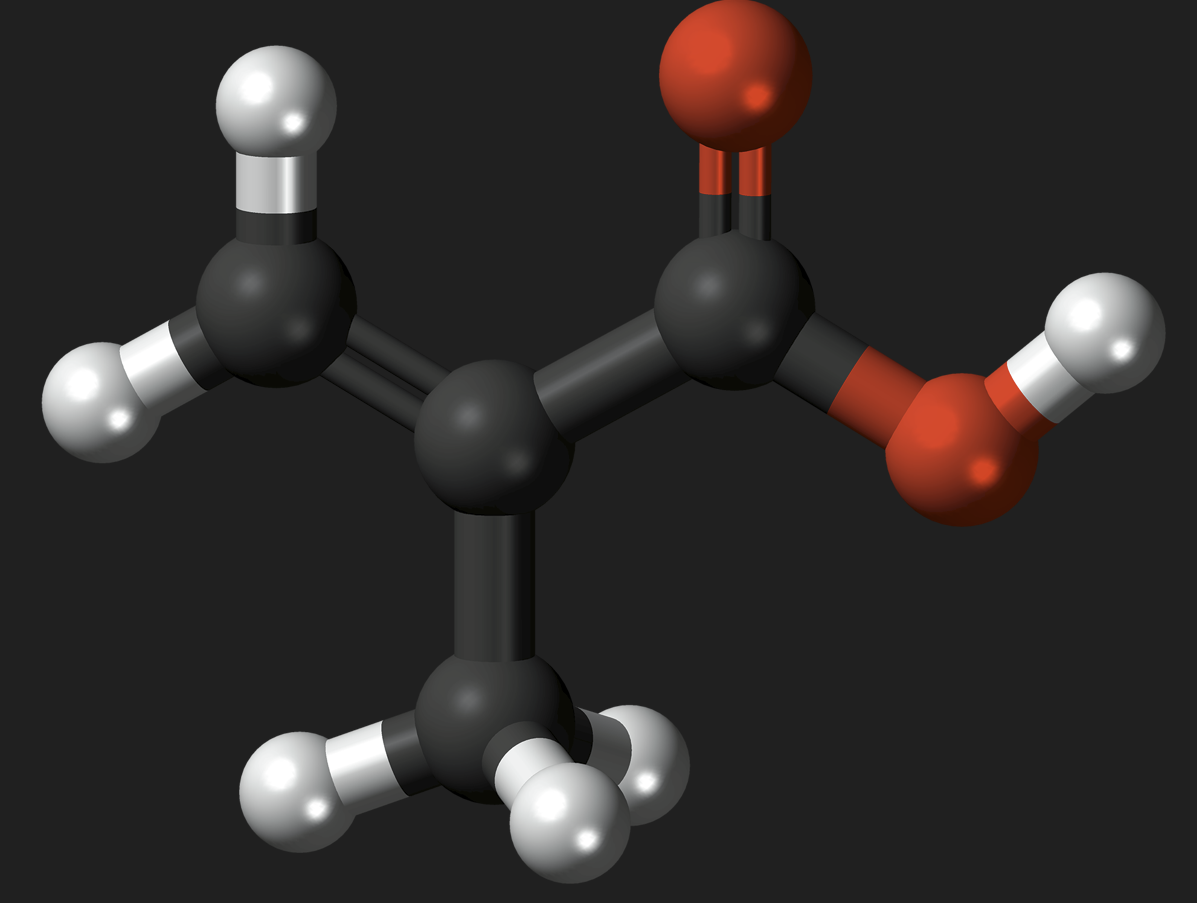
Acrylic acid and methacrylic acid are pivotal in the world of industrial chemistry, serving as foundational elements in the production of numerous products we encounter daily. From adhesives to paints and healthcare applications, these substances play crucial roles. Though often discussed in tandem due to their similar molecular structures, the distinct differences between these acids significantly impact their usage and handling.
Acrylic acid is a clear, colorless liquid known for its acrid odor and propensity to bond easily with various substrates, forming polymers. Methacrylic acid, on the other hand, is characterized by a slightly different molecular structure that confers extra resistance to heat and weather conditions, making it ideal for applications requiring greater durability. Both acids are essential in polymer manufacturing but are used differently based on their physical and chemical properties.
Their significance extends beyond simple polymerization. Acrylic acid is predominantly utilized in products requiring flexibility, like diapers and adhesives, while methacrylic acid features prominently in products that must withstand harsh environments, such as outdoor paints and dental materials. Understanding these differences is crucial for leveraging their properties in industrial applications effectively.
Chemical Properties
Basic Chemical Structure of Acrylic Acid
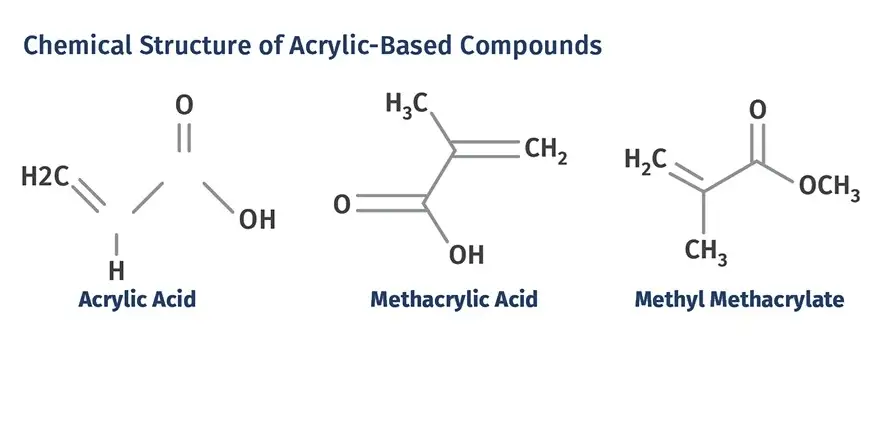
Acrylic acid, scientifically known as propenoic acid, has a simple chemical structure characterized by its vinyl group, making it an alkenoic acid. Its molecular formula is C3H4O2, and it consists of a carboxylic group attached to an ethylene group, which allows it to readily participate in addition reactions, a feature crucial to its role in polymerization processes.
Basic Chemical Structure of Methacrylic Acid
Methacrylic acid, or 2-methylpropenoic acid, shares a similar structural base with acrylic acid but features an additional methyl group on the alpha carbon of the vinyl group. This structure, represented by the molecular formula C4H6O2, significantly impacts its chemical behavior and reactivity compared to its acrylic counterpart.
Comparison of Their Molecular Properties
Both acids exhibit high reactivity due to their double bonds and carboxylic groups. However, the presence of the methyl group in methacrylic acid increases its steric hindrance, making it less reactive towards certain types of polymerization and more resistant to degradation under heat and light. This subtle difference marks a significant divergence in their applications and processing.
Production Methods
How Acrylic Acid is Produced
- Oxidation of Propylene: This is the most common method for producing acrylic acid. It involves the catalytic partial oxidation of propylene, a byproduct of oil refining and natural gas processing. The process requires precise control over temperature and pressure to maximize yield and minimize byproducts.
How Methacrylic Acid is Produced
- Acetone Cyanohydrin Process: Methacrylic acid production predominantly uses this method, where acetone and hydrogen cyanide are converted into acetone cyanohydrin, which is subsequently processed with sulfuric acid to produce methacrylamide sulfate. This is then hydrolyzed under controlled conditions to yield methacrylic acid.
Differences in Production Processes
- Raw Materials: Acrylic acid relies on propylene, while methacrylic acid starts from acetone, pointing to different dependencies on raw material markets.
- Complexity and Yield: The acetone cyanohydrin process is more complex and involves more steps than the direct oxidation of propylene, influencing both cost and scalability.
Applications
Common Applications of Acrylic Acid
Acrylic acid is primarily used in the production of superabsorbent polymers found in products like baby diapers and adult incontinence pads. It is also a key component in water-based acrylic paints and coatings, which are valued for their environmental friendliness and ease of application.
Common Applications of Methacrylic Acid
Methacrylic acid is crucial in the manufacture of polymethyl methacrylate (PMMA), used extensively in the automotive and construction industries for its clarity and durability. It also plays a significant role in the production of dental and orthopedic cements, showcasing its utility in medical applications.
Unique Uses in Industry
- Acrylic Acid: In agriculture, acrylic polymers are used as soil conditioners to improve water retention and aeration.
- Methacrylic Acid: It is used in the manufacturing of LED light panels, where its optical clarity and resistance to weathering are essential.
Physical Properties
Boiling Points and Melting Points
Acrylic acid has a boiling point of approximately 141°C and a melting point of 13°C, whereas methacrylic acid boils around 160°C and melts at 15°C. These thermal properties affect how they are stored and handled in industrial settings.
Viscosity and Solubility
- Acrylic Acid: It is relatively low in viscosity and highly soluble in water, which facilitates its use in aqueous solutions for coatings and adhesives.
- Methacrylic Acid: Although also water-soluble, it has a slightly higher viscosity, which can influence its handling in polymer synthesis.
How These Properties Affect Usage
The lower boiling point of acrylic acid makes it more volatile and requires careful handling and storage conditions. In contrast, methacrylic acid’s higher resistance to heat makes it suitable for applications requiring prolonged exposure to high temperatures, such as in automotive plastics and exterior paints.
Reactivity and Safety
Reactivity with Common Substances
Acrylic and methacrylic acids are both highly reactive due to their unsaturated carboxylic acid structure. Acrylic acid can polymerize explosively when exposed to heat, light, or peroxides. Similarly, methacrylic acid is sensitive to light and heat, although its additional methyl group provides a bit more stability compared to acrylic acid. Both acids react strongly with bases and can undergo hazardous reactions if improperly mixed with oxidizing agents.
Safety Measures for Handling Acrylic Acid
Handling acrylic acid requires strict safety protocols to prevent accidents:
- Use of Personal Protective Equipment (PPE): Gloves, goggles, and protective clothing are essential to prevent skin and eye contact.
- Proper Ventilation: Work areas should be well-ventilated to avoid inhalation of fumes.
- Storage Conditions: Acrylic acid should be stored in cool, dry places away from direct sunlight and heat sources to prevent polymerization.
Safety Measures for Handling Methacrylic Acid
Methacrylic acid also requires careful handling:
- Spill Management: Immediate cleanup of spills with appropriate neutralizing agents to prevent environmental release and health hazards.
- Fire Safety: Use of carbon dioxide or dry chemical fire extinguishers to handle fires, as water can cause the spread of acid.
- Exposure Controls: Installation of emergency showers and eyewash stations near workstations.
Environmental Impact
Biodegradability of Each Acid
Both acrylic and methacrylic acid are biodegradable under aerobic conditions, which means they can be broken down by microorganisms when exposed to air. However, the rate and completeness of biodegradation can vary based on environmental conditions and microbial populations.
Environmental Risks Associated with Each
The primary environmental concern with these acids involves their toxicity to aquatic life. Both acids can decrease pH in water bodies, leading to harmful effects on aquatic organisms and ecosystems. Leaks and spills must be managed quickly to prevent such impacts.
Regulations and Safety Standards
Numerous international standards and regulations govern the handling, transportation, and disposal of acrylic and methacrylic acids. In the United States, OSHA and the EPA provide guidelines to ensure worker safety and environmental protection. Globally, the REACH regulation in Europe monitors and controls chemical risks.
Economic Impact
Global Market Trends for Acrylic Acid
The global market for acrylic acid has seen steady growth, driven by demand in superabsorbent polymers and adhesives. Asia-Pacific regions, particularly China and India, are leading this expansion due to rapid industrialization and increased consumer product demand.
Global Market Trends for Methacrylic Acid
Similarly, methacrylic acid markets are expanding, fueled by demand in the automotive and electronics industries. Innovations in PMMA applications, such as LED diffusers and automotive lights, are pushing growth in this sector.
Factors Influencing Market Dynamics
Key factors influencing the dynamics of both markets include:
- Raw Material Availability: Fluctuations in the supply of propylene and acetone can affect production costs.
- Technological Advances: Innovations in production processes can improve yields and reduce waste.
- Regulatory Changes: Environmental and safety regulations can drive changes in manufacturing and application sectors.
Future Prospects
Recent Developments in the Use of Each Acid
Recent advances include the development of more efficient catalysts for the production of both acids, reducing energy consumption and increasing yield. New polymer blends utilizing acrylic and methacrylic acids are being developed for medical and environmental applications.
Potential Future Applications and Innovations
Research is ongoing in the use of acrylic and methacrylic acids in renewable energy applications, including solar panels and fuel cells. These acids are being explored for their potential to improve the efficiency and durability of these technologies.
Challenges and Opportunities in the Market
The main challenges facing these markets include handling the volatility in raw material prices and adhering to strict environmental regulations. However, these challenges also present opportunities for innovation in recycling technologies and the development of bio-based alternatives, which could lead to more sustainable and environmentally friendly products.
Frequently Asked Questions
What is Acrylic Acid?
Acrylic acid (propenoic acid) is a versatile chemical primarily used in the production of acrylate polymers. It’s a key ingredient in superabsorbent polymers, adhesives, and coatings due to its excellent binding characteristics and fast-drying nature.
What is Methacrylic Acid?
Methacrylic acid is a chemical compound used mainly to produce methacrylate polymers and copolymers. It is highly valued for its increased resistance to heat and light, making it suitable for applications in the automotive and dental industries.
How are Acrylic and Methacrylic Acids Produced?
Acrylic acid is typically produced by the oxidation of propylene, a byproduct of gasoline refining, whereas methacrylic acid is produced through the acetone cyanohydrin process, involving different raw materials and conditions.
What Are the Safety Concerns with These Acids?
Both acrylic and methacrylic acids are corrosive and can cause severe burns upon contact with skin or eyes. Proper handling, including the use of safety gear and adherence to safety protocols, is imperative to prevent accidents.
Can Acrylic and Methacrylic Acids be Recycled?
While recycling these acids is complex, research into catalysts and processes may soon make it possible to reclaim them from polymer waste, presenting an opportunity to reduce environmental impact and reclaim valuable materials.
Conclusion
The differences between acrylic and methacrylic acids underscore the diversity of applications and the importance of chemical specificity in industrial processes. While both serve as building blocks for polymers, their distinct properties dictate their suitability for different applications, from everyday items to specialized industrial uses. Understanding these subtleties not only enhances safety and efficiency in their use but also opens up avenues for innovation in product development. As the industry continues to evolve, the roles of acrylic and methacrylic acids are likely to expand, driven by advances in chemistry and material sciences.