Oxides, compounds formed by the reaction of oxygen with other elements, occupy a pivotal place in both the natural world and industrial applications. Their ubiquity ranges from the rust that forms on iron to the oxygen we breathe, combined with carbon in carbon dioxide. Differentiating between metal and nonmetal oxides is crucial due to their distinct properties and reactions with other substances, influencing countless processes from manufacturing to environmental chemistry.
Metal oxides tend to be basic, reacting with acids to form salt and water, while nonmetal oxides are generally acidic, reacting with bases to produce salts and water. This fundamental difference underscores the diverse chemical behaviors and applications of oxides in various domains, including technology, manufacturing, and environmental management.
In exploring oxides, we delve into the realm of chemistry where the interaction between elemental oxygen and a plethora of other elements gives rise to compounds with a wide array of properties. From their role in the earth’s crust as major constituents of minerals to their applications in catalysis, electronics, and pollution control, the study of metal and nonmetal oxides opens a window to understanding the material basis of our world and the innovative ways humans have harnessed their potential.
Metal Oxides
Definition and Examples
Metal oxides are compounds consisting of oxygen atoms bonded to metal atoms. Common examples include iron oxide (Fe2O3), known as rust, and magnesium oxide (MgO), a white powder with extensive uses.
Key Characteristics
Metal oxides are generally:
- Basic in nature
- High melting and boiling points
- Often solid at room temperature
Common Uses and Applications
Metal oxides play a crucial role in various industries:
- Catalysis: For speeding up chemical reactions
- Electronics: As semiconductors and insulators
- Construction: In making glasses and ceramics
Nonmetal Oxides
Definition and Examples
Nonmetal oxides form when oxygen combines with nonmetal elements. Examples include carbon dioxide (CO2) and sulfur dioxide (SO2), both key to environmental processes.
Key Characteristics
Nonmetal oxides are typically:
- Acidic in nature
- Gases or volatile liquids at room temperature
- Important in environmental chemistry
Common Uses and Applications
- Purification: Treating water to remove impurities
- Manufacturing: Producing acids for industrial use
- Environmental Monitoring: Tracking air quality and pollution
Chemical Properties
Reaction with Water
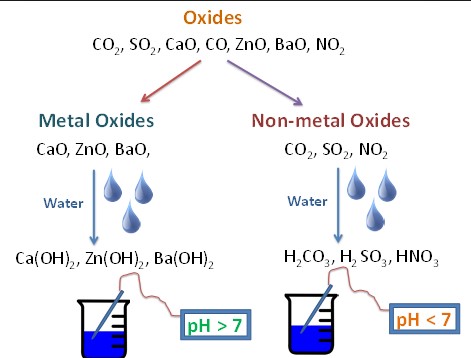
Metal Oxides Reactions
When mixed with water, many metal oxides form bases or alkaline solutions. For instance:
- Magnesium oxide + water -> Magnesium hydroxide
Nonmetal Oxides Reactions
Nonmetal oxides react with water to form acidic solutions. For example:
- Carbon dioxide + water -> Carbonic acid
Reaction with Acids
Metal Oxides Reactions
Metal oxides react with acids to produce salt and water, a neutralization reaction. Such as:
- Calcium oxide + hydrochloric acid -> Calcium chloride + water
Nonmetal Oxides Reactions
Nonmetal oxides do not typically react with acids due to their already acidic nature.
Reaction with Bases
Metal Oxides Reactions
Some metal oxides can react with bases in a process called neutralization, though less common.
Nonmetal Oxides Reactions
Nonmetal oxides react with bases to form salts and water, highlighting their acidic properties. Example:
- Sulfur dioxide + sodium hydroxide -> Sodium sulfite + water
Physical Properties
Color and Form
Metal oxides are usually solid, often colorful or metallic. Nonmetal oxides, on the other hand, are often gases or colorless liquids.
Melting and Boiling Points
Metal oxides boast high melting and boiling points. Nonmetal oxides vary widely, with many being gaseous at room temperature.
Solubility in Water
- Metal oxides: Some are soluble, forming alkaline solutions.
- Nonmetal oxides: Generally soluble, creating acidic solutions.
Environmental Impact
Metal Oxides
Positive and Negative Impacts
- Positive: Used in pollution control devices.
- Negative: Certain metal oxides can be toxic or lead to environmental degradation when improperly managed.
Nonmetal Oxides
Positive and Negative Impacts
- Positive: Essential in natural processes, like photosynthesis.
- Negative: Contribute to acid rain and greenhouse effect, posing challenges to environmental health.
Industrial and Practical Applications
Metal Oxides
Metal oxides serve a variety of functions across multiple industries, underscoring their versatility and critical role in modern technology and manufacturing.
Specific Uses in Industries
- Electronics and Semiconductors: Silicon dioxide (SiO2) is foundational in the manufacturing of semiconductor devices.
- Catalysis: Vanadium(V) oxide (V2O5) is used in sulfuric acid production and in the reduction of nitrogen oxides in industrial emissions.
- Ceramics and Glass: Aluminum oxide (Al2O3) and silicon dioxide are integral in producing refractory materials, glass, and ceramics due to their high melting points and mechanical strength.
Nonmetal Oxides
Nonmetal oxides are equally significant, finding application in various sectors, particularly in environmental management and chemical synthesis.
Specific Uses in Industries
- Environmental Monitoring and Control: Sulfur dioxide (SO2) and nitrogen oxides (NOx) are monitored for air quality management and in the development of strategies to reduce acid rain.
- Chemical Industry: Carbon dioxide (CO2) is utilized in the production of carbonated beverages and is a key reactant in the synthesis of urea and methanol.
Health and Safety Considerations
Exposure Risks
Metal Oxides vs. Nonmetal Oxides
- Metal Oxides: Exposure can lead to respiratory issues, skin irritation, and in the case of heavy metals, more severe health effects.
- Nonmetal Oxides: Pose risks primarily through inhalation, potentially leading to respiratory problems, and in the case of CO2, elevated levels can result in asphyxiation.
Handling and Storage
Best Practices
- Proper Ventilation: Ensure areas are well-ventilated to dilute and remove airborne particles or gases.
- Protective Gear: Use appropriate personal protective equipment (PPE), such as gloves, goggles, and masks.
- Storage Requirements: Store in appropriately labeled, sealed containers in controlled environments to prevent accidental exposure or reaction.
Recent Advances and Research
Innovations in the Use of Oxides
Recent years have seen remarkable innovations in the application and understanding of both metal and nonmetal oxides.
- Energy Storage: Research into metal oxides, especially lithium oxide, is pushing the boundaries of battery technology, offering prospects for higher capacity and longer life batteries.
- Environmental Purification: Nonmetal oxides are at the forefront of developing more efficient methods of carbon capture and air purification, aiming to mitigate the impact of greenhouse gases.
Future Prospects
The future holds promising potential for the development of new materials and technologies leveraging the unique properties of oxides.
- Nanotechnology: Both metal and nonmetal oxides are pivotal in the creation of nanomaterials for use in medicine, electronics, and energy.
- Sustainable Practices: Advances in oxide research are leading toward more sustainable industrial processes, reducing environmental impact and enhancing efficiency.
Frequently Asked Questions
What are metal oxides?
Metal oxides are compounds composed of metal elements combined with oxygen. They typically exhibit basic properties, meaning they can react with acids to form salts and water. Metal oxides are prevalent in various industrial processes, serving as catalysts, pigments, and in the manufacture of ceramics and glass.
How do nonmetal oxides differ from metal oxides?
Nonmetal oxides differ from metal oxides primarily in their chemical properties; they are usually acidic, reacting with bases to produce salts and water. This characteristic makes nonmetal oxides crucial in many environmental and chemical processes, including the formation of acid rain from sulfur and nitrogen oxides in the atmosphere.
Can oxides be both acidic and basic?
Yes, certain oxides, known as amphoteric oxides, exhibit both acidic and basic properties. They can react with both acids and bases to form salts and water. Examples include zinc oxide and aluminum oxide. This dual nature allows them to play versatile roles in chemical reactions and industrial applications.
What role do oxides play in the environment?
Oxides play significant roles in the environment, with metal oxides being integral to the earth’s crust and nonmetal oxides contributing to phenomena such as acid rain and the greenhouse effect. Their interactions with natural systems are critical to understanding environmental chemistry, pollution, and strategies for sustainable living.
Conclusion
Oxides, forming the bridge between the elemental and the compound, are fundamental to both our understanding of chemistry and our ability to leverage chemical processes for technological, environmental, and industrial purposes. The distinction between metal and nonmetal oxides is not just academic; it informs countless applications and innovations, from the creation of new materials to the mitigation of environmental impacts.
As we continue to explore and manipulate the properties of these substances, the knowledge of their differences and similarities remains a cornerstone of progress. In this quest, the study of metal and nonmetal oxides exemplifies the blend of curiosity, understanding, and application that drives the scientific endeavor forward, promising new discoveries and improvements for the quality of human life and the health of our planet.

