Quantum numbers play a pivotal role in the realm of quantum mechanics, acting as the unique identifiers that define the state of an electron within an atom. These numbers not only help in understanding the electron’s behavior but also its interaction with magnetic fields and other electrons. Among the four quantum numbers, the magnetic quantum number and the spin quantum number are crucial for delineating the orientation and spin of an electron, respectively.
The magnetic quantum number (m) indicates the orientation of an electron’s orbital around the nucleus, while the spin quantum number (s) reveals the direction of the electron’s spin. In essence, while both relate to an electron’s position and movement, they focus on different aspects: one on the spatial orientation in an external magnetic field, and the other on the intrinsic spin property of the electron itself.
Quantum mechanics is not just a theoretical construct but a foundation for modern physics and technology, influencing everything from chemical bonding to the operation of quantum computers. The differentiation between the magnetic and spin quantum numbers is not just academic; it has practical implications in understanding atomic structures, chemical reactions, and advanced technological applications.
Quantum Numbers Explained
Basics of Quantum Numbers
Definition and Role
Quantum numbers are fundamental to the study of quantum mechanics, providing a comprehensive system to describe the unique state of an electron in an atom. These numbers detail the electron’s properties, including its energy level, orbital shape, orbital orientation, and spin direction. Essentially, quantum numbers allow scientists to predict an electron’s behavior and how it interacts with other particles and fields.
Types of Quantum Numbers
There are four main quantum numbers:
- Principal quantum number (n): Indicates the energy level of an electron.
- Azimuthal quantum number (l): Determines the shape of the electron’s orbital.
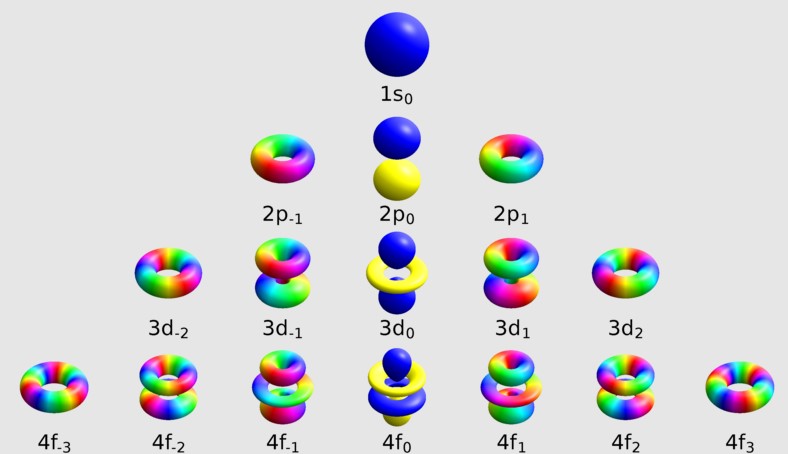
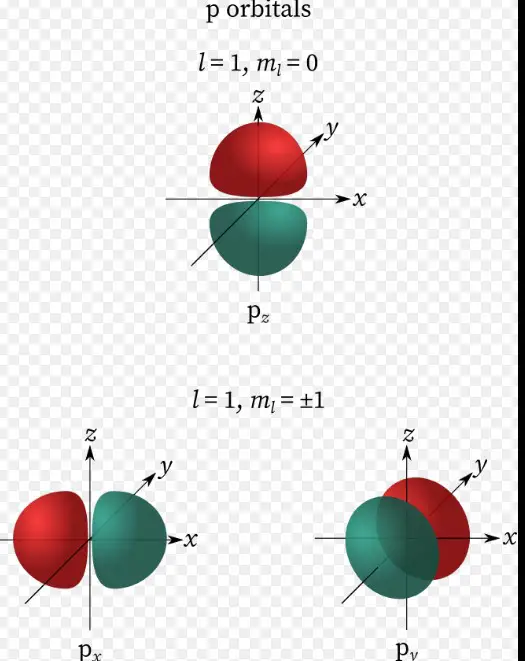
- Magnetic quantum number (m): Describes the orientation of the orbital in space.
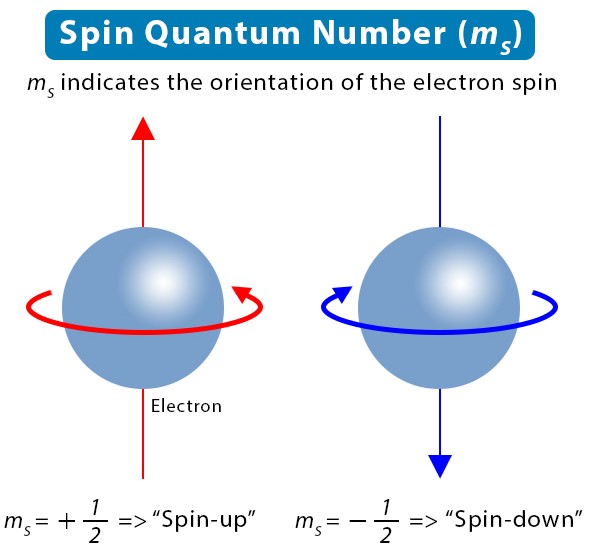
- Spin quantum number (s): Specifies the direction of the electron’s spin.
Focus on Magnetic and Spin Quantum Numbers
Introduction to Magnetic Quantum Number (m)
The magnetic quantum number, denoted as m, is crucial for understanding an electron’s orientation in three-dimensional space. It explains how orbitals are aligned in the presence of a magnetic field, directly influencing the electron’s energy state within an atom.
Introduction to Spin Quantum Number (s)
The spin quantum number, represented as s, reveals the intrinsic spin of an electron. Unlike the other quantum numbers, s relates to an electron’s fundamental property rather than its position or movement within an atom. It’s key to understanding the magnetic properties of atoms and molecules.
Magnetic Quantum Number
Overview
Definition
The magnetic quantum number defines the orbital’s orientation around the nucleus, crucial for the spatial arrangement of electrons.
Relation to Orbital Angular Momentum
M is directly related to the azimuthal quantum number (l), reflecting how angular momentum influences an electron’s magnetic energy.
Characteristics
Range and Values
- M can take on any value from -l to +l, including zero.
- This range determines the number of orbitals at a given energy level, influencing the electron distribution.
Determination Process
- M is calculated based on the electron’s energy level and orbital shape.
- The selection of m values is governed by the azimuthal quantum number (l).
Significance
Influence on Electron Orientation
- M significantly impacts how electrons are aligned within an atom, affecting chemical bonding and molecular structure.
Role in Atomic Structure
- It determines the multiplicity of electron states within an orbital, essential for spectroscopy and quantum chemistry.
Spin Quantum Number
Overview
Definition
The spin quantum number identifies the direction of an electron’s spin, a quantum mechanical property that contributes to the magnetic field generated by the electron.
Fundamental Properties
- Spin is an inherent characteristic of particles, akin to mass or charge.
- S can have a value of +1/2 or -1/2, indicating the two possible spin states of an electron.
Characteristics
Possible Values
- S distinguishes electrons in the same orbital, with values pointing to either “up” or “down” spin orientations.
Physical Interpretation
- The spin of an electron is a quantum phenomenon, not visualizable in classical terms but essential for understanding magnetic interactions.
Significance
Representation of Electron Spin
- S is pivotal for quantum mechanics, affecting everything from atomic structure to magnetic resonance imaging (MRI).
Impact on Electron Pairing and Magnetism
- Electrons with opposite spins can occupy the same orbital, a principle underlying the Pauli exclusion principle and chemical bonding.
- The collective spins of electrons contribute to a material’s magnetic properties, influencing its behavior in magnetic fields.
Key Differences
Comparison Table
Let’s start with a quick reference guide to highlight the fundamental differences between magnetic quantum numbers (m) and spin quantum numbers (s):
| Feature | Magnetic Quantum Number (m) | Spin Quantum Number (s) |
|---|---|---|
| Definition | Orientation of an electron’s orbital around the nucleus | Direction of the electron’s intrinsic spin |
| Range of Values | -l to +l, where l is the azimuthal quantum number | -1/2, +1/2 |
| Physical Meaning | Spatial orientation of an electron’s probability cloud within an atom | Intrinsic angular momentum of an electron |
| Influence on Behavior | Determines the electron’s path around the nucleus | Affects the magnetic and spin properties of the electron |
| Role in Atomic Structure | Affects the shape and orientation of atomic orbitals | Essential for understanding electron pairing and magnetism |
Detailed Analysis
Conceptual Distinction
The conceptual distinction between the magnetic and spin quantum numbers lies in their respective focuses on spatial orientation versus intrinsic properties. The magnetic quantum number (m) maps out the electron’s orbit around the nucleus, integral for understanding the electron’s position within an atom. In contrast, the spin quantum number (s) delves into the electron’s own inherent spin characteristic, a quantum feature that has no classical analog.
Impact on Electron Behavior
The impact on electron behavior is pronounced for both quantum numbers. The magnetic quantum number influences how electrons are distributed around an atom’s nucleus, affecting the atom’s energy levels and chemical bonding potential. On the other hand, the spin quantum number dictates how electrons pair within orbitals, fundamental to the Pauli exclusion principle and the magnetic properties of materials.
Role in Magnetic Properties
The role in magnetic properties is primarily governed by the spin quantum number, as it directly influences an electron’s magnetic orientation. This, in turn, affects how atoms interact with external magnetic fields, critical for technologies like MRI and magnetic storage devices. The magnetic quantum number, while indirectly influencing magnetic properties through spatial orientation, primarily affects the electron’s energy levels and orbital shapes.
Applications and Implications
In Atomic Physics
Electron Configuration
Understanding the electron configuration of atoms is crucial in atomic physics. The magnetic and spin quantum numbers provide a detailed blueprint of how electrons are arranged around an atom’s nucleus. This arrangement not only determines the atom’s chemical properties but also its reactivity, bonding capacity, and spectral characteristics.
Atomic Spectra Analysis
The analysis of atomic spectra, the fingerprints of atoms, relies heavily on these quantum numbers. The magnetic quantum number helps explain the splitting of spectral lines in a magnetic field (Zeeman effect), while the spin quantum number is key to understanding fine structures in spectral lines due to spin-orbit coupling.
In Quantum Mechanics
Quantum State Determination
In quantum mechanics, the complete quantum state of an electron is determined by all four quantum numbers, including the magnetic and spin quantum numbers. These numbers are essential for solving Schrödinger’s equation for atoms, predicting the probability distributions of electrons, and understanding the quantum behaviors of particles.
Spin-Orbit Interaction
The spin-orbit interaction, a crucial concept in quantum mechanics, describes the interaction between an electron’s spin and its orbital motion around the nucleus. This phenomenon, influenced by both quantum numbers, leads to energy level splitting and is pivotal in explaining atomic structure, chemical bonding, and the color of compounds.
In Technology
MRI Technology
MRI (Magnetic Resonance Imaging) technology exploits the principles of nuclear magnetic resonance, which is directly related to the spin quantum number of particles. By aligning and then disturbing the spin orientation of hydrogen nuclei in water molecules within the body, MRI machines can create detailed images of internal structures, showcasing the practical application of quantum numbers in medical diagnostics.
Quantum Computing
Quantum computing, a frontier technology, leverages the principles of quantum mechanics, including the fundamental role of spin quantum numbers. Qubits, the basic units of quantum information, often use the spin states of particles as a means to represent and manipulate data, promising unprecedented computational speed and power for solving complex problems.
FAQs
What are quantum numbers?
Quantum numbers are numerical values that describe the unique state of an electron in an atom. They include the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (m), and the spin quantum number (s). Each provides information about the electron’s energy level, shape of the orbital, orientation, and spin, respectively.
How does the magnetic quantum number differ from the spin quantum number?
The magnetic quantum number identifies the orientation of an electron’s orbital in a magnetic field, offering insights into the spatial distribution around the nucleus. In contrast, the spin quantum number relates to the electron’s intrinsic spin, indicating its rotation direction. While both contribute to defining an electron’s state, they address distinct characteristics: orbital orientation versus spin.
Why are these quantum numbers important?
Understanding the magnetic and spin quantum numbers is crucial for predicting the chemical and physical properties of atoms and molecules. They influence magnetic resonance imaging (MRI), electron configurations, and the behavior of materials in magnetic fields. Moreover, these quantum numbers are foundational in quantum chemistry and physics, supporting advancements in technology and materials science.
Conclusion
The distinctions between the magnetic quantum number and the spin quantum number are fundamental to the study and application of quantum mechanics. Their roles in determining the orientation and spin of electrons within atoms not only enrich our understanding of atomic and molecular structures but also pave the way for innovations in technology and science.
Recognizing the difference and significance of these quantum numbers enhances our comprehension of the microscopic world, influencing the development of new technologies and materials. As the boundaries of quantum mechanics expand, the importance of these quantum numbers in both theoretical and practical domains continues to grow, underscoring the interconnectedness of science and technology.