Molecular structures play a pivotal role in the fields of chemistry and physics, affecting everything from the physical properties of materials to the behavior of chemicals. At the core of this influence are the shapes of molecules, specifically whether they are linear or nonlinear. These shapes not only define the identity of a molecule but also its functionality and interactions in various environments.
The difference between linear and nonlinear molecules is primarily based on their geometric arrangement. Linear molecules have all their atoms arranged in a straight line, with bond angles of 180 degrees. In contrast, nonlinear molecules feature atoms arranged in an angular or bent configuration, leading to diverse molecular shapes and varying bond angles.
Molecular geometry is crucial as it influences chemical reactivity, polarity, phase of matter, and even color. Understanding whether a molecule is linear or nonlinear helps predict these properties, providing invaluable insight for applications ranging from industrial manufacturing to pharmaceuticals.
Basic Concepts
What Are Molecules?
Molecules are the smallest units of a chemical compound that can exist independently while retaining all the chemical properties of the compound. They are composed of two or more atoms bonded together through chemical bonds. These bonds form as a result of the sharing or exchange of electrons among atoms.
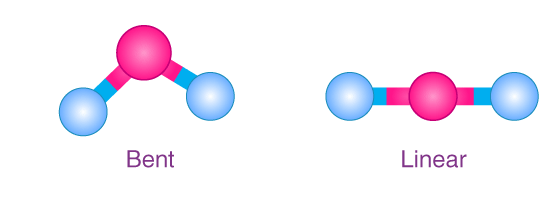
Linear vs. Nonlinear Definitions
Linear molecules are characterized by a straight configuration of atoms. In these molecules, atoms are aligned in a single straight line, often with bond angles fixed at 180 degrees. Nonlinear molecules, on the other hand, do not follow a straight line. Their atoms are arranged in various angular formations, leading to multiple bond angles that are not 180 degrees.
Linear Molecules
Characteristics
Linear molecules are distinguished by their symmetrical shape and uniform bond angles. The symmetry often results in simplified mathematical models for describing their behavior, particularly in physical phenomena like diffusion or spectroscopy.
Common Examples
A familiar example of a linear molecule is carbon dioxide (CO2), where the oxygen atoms are on opposite sides of the carbon, forming a straight line. Another example is acetylene (C2H2), a gas used in welding, which also displays a linear arrangement of its carbon and hydrogen atoms.
Applications in Industry
Linear molecules have significant applications in industry:
- Manufacturing: Many polymers, like polyethylene, involve linear molecular structures that contribute to their flexibility and strength.
- Aerospace: Rocket propellants often use linear molecules such as hydrazine, which provide high energy densities ideal for space missions.
Nonlinear Molecules
Key Features
Nonlinear molecules are noted for their complex shapes due to various angular bonds. This complexity often leads to interesting chemical and physical properties, such as higher reactivity and diverse molecular interactions.
Typical Examples
Water (H2O) is perhaps the most well-known nonlinear molecule, with a bent shape due to its two hydrogen atoms being displaced from a straight alignment with the oxygen. Ammonia (NH3) also has a nonlinear shape, with its three hydrogen atoms forming a pyramid around the nitrogen atom.
Uses in Technology
Nonlinear molecules are crucial in numerous technological applications:
- Electronics: Nonlinear optical materials are used for creating components in lasers and telecommunications.
- Pharmaceuticals: Many drugs rely on the unique shapes of nonlinear molecules to bind specifically to targets in the body.
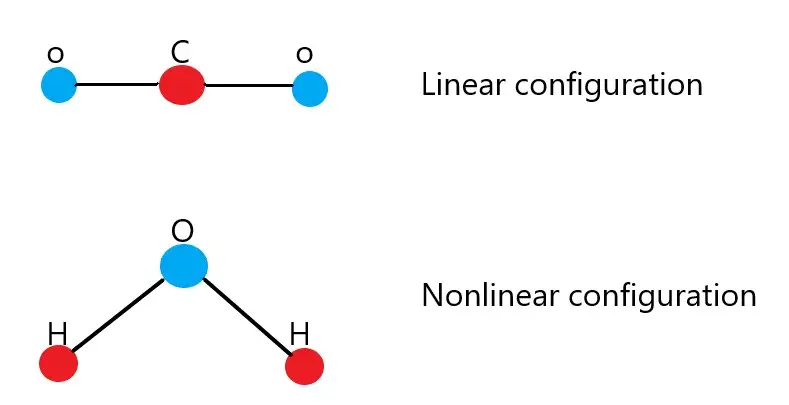
Structural Comparison
Bond Angles and Symmetry
The bond angles in linear molecules are typically at 180 degrees, creating symmetrical structures that are often less reactive chemically. In contrast, nonlinear molecules have varying bond angles, leading to asymmetry and potentially more dynamic chemical behaviors.
Energy States
The energy states of molecules are influenced by their structure. Linear molecules generally have more predictable and simpler energy states compared to nonlinear molecules, whose irregular shapes introduce complexity in their energy landscapes. This complexity can affect how these molecules absorb light, interact with other molecules, and participate in chemical reactions.
Impact on Properties
Physical Properties
The structural differences between linear and nonlinear molecules greatly influence their physical properties, such as boiling and melting points, viscosity, and solubility. Linear molecules, due to their symmetrical shape, often have lower boiling points compared to nonlinear molecules of similar molar masses. This is due to the more compact and organized way in which linear molecules can pack together, leading to weaker intermolecular forces.
Chemical Reactivity
Chemical reactivity is also heavily influenced by molecular geometry. Nonlinear molecules typically exhibit higher reactivity than linear ones. This increased reactivity can be attributed to the presence of more complex bonding arrangements and the ability to participate in multiple chemical interactions. For example, the bent shape of water allows it to be a universal solvent, interacting with various other molecules through hydrogen bonding.
Visualization Techniques
To better understand and analyze the structure of molecules, scientists employ various visualization techniques. These methods provide critical insights into how molecular structures influence their properties and behaviors.
Spectroscopy
Spectroscopy is a key technique used to observe the interaction of molecules with electromagnetic radiation. There are several types of spectroscopy, each useful for different kinds of molecules and molecular features:
- Infrared (IR) Spectroscopy: Useful for detecting functional groups in organic compounds.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: Offers detailed information about the structure, dynamics, reaction state, and chemical environment of molecules.
- Ultraviolet-visible (UV-Vis) Spectroscopy: Applied primarily to analyze compounds with conjugated systems.
X-ray Crystallography
X-ray crystallography is another powerful technique used to determine the atomic and molecular structure of a crystal. By measuring the angles and intensities of X-rays scattered by the crystal, scientists can map out the electron density and infer the positions of the atoms within the material. This method is particularly effective for complex molecules, where the exact details of bonding and structure are critical, such as in pharmaceuticals and fine chemicals.
Practical Applications
In Pharmaceuticals
The shape of molecules is critical in the development of pharmaceuticals. Drugs must be designed to interact precisely with biological targets, which often requires a specific molecular shape. For example, the nonlinear structure of many drugs allows them to bind more effectively to proteins, blocking or promoting biochemical pathways.
In Material Science
In the field of material science, the properties imparted by molecular structure are exploited to create materials with specific characteristics. Polymers, for instance, might be designed with a particular molecular architecture to enhance strength, flexibility, or resistance to degradation.
Challenges and Solutions
Analytical Difficulties
Analyzing molecular structures presents several challenges, particularly when dealing with complex molecules that have multiple functional groups or stereocenters. Traditional methods like NMR and IR spectroscopy can sometimes provide limited information on such structures.
Advances in Molecular Modeling
To overcome these challenges, advances in molecular modeling have been crucial. Computational techniques, including quantum mechanical modeling and molecular dynamics simulations, allow scientists to visualize and predict the behavior of molecules in ways that are not possible with experimental methods alone. These tools are invaluable for the design of new materials and drugs, offering insights into molecular interactions at the atomic level.
FAQs
What defines a linear molecule?
Linear molecules are characterized by a straight arrangement of atoms with no angular deviations, typically involving diatomic molecules or those with a central atom double-bonded to others, leading to a straight structure.
How do nonlinear molecules differ?
Nonlinear molecules differ by having bent or angular structures, which can result in varying bond angles that are not 180 degrees. This variation often leads to more complex molecular behaviors and properties.
Why does molecular shape matter?
The shape of a molecule directly impacts its chemical properties and interactions. For instance, molecular polarity and the ability to form certain chemical bonds are influenced by molecular geometry.
What are examples of linear molecules?
Common examples of linear molecules include carbon dioxide (CO2) and acetylene (C2H2), where atoms are aligned in a straight line.
What are examples of nonlinear molecules?
Water (H2O) and ammonia (NH3) are typical examples of nonlinear molecules, featuring bent and trigonal pyramidal shapes, respectively.
Conclusion
Understanding the distinctions between linear and nonlinear molecules enriches our comprehension of chemical and physical behaviors across various substances. The implications of these differences are vast, affecting everything from the basic understanding of chemical interactions to practical applications in designing materials and drugs.
As advancements in technology allow for more detailed explorations of molecular structures, the insights gained from studying these differences will undoubtedly continue to contribute significantly to scientific progress and industrial innovation. This knowledge not only enhances academic pursuits but also supports real-world applications in myriad industries.