Chemical synthesis lies at the heart of modern scientific endeavors, bridging the gap between theoretical chemistry and practical applications in materials science, pharmaceuticals, and beyond. This intricate process involves the step-by-step construction of complex molecules from simpler substances, employing strategies that significantly influence the efficiency, yield, and scalability of the production.
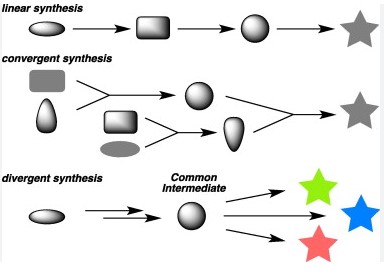
The core difference between linear and convergent synthesis is rooted in their approach to molecule assembly. Linear synthesis involves constructing a molecule step by step in a sequential manner, often leading to a build-up of intermediates. Conversely, convergent synthesis takes a more parallel approach, where larger pre-assembled sections of the molecule are combined, aiming to reduce the number of steps and intermediates involved.
Both strategies play pivotal roles in the synthesis of complex molecules, yet they cater to different needs and scenarios in chemical manufacturing and research. The choice between linear and convergent synthesis depends on various factors, including the complexity of the molecule, desired yield, and production scalability. Understanding their distinct advantages and limitations is crucial for chemists and researchers looking to optimize their synthetic routes.
Basics of Synthesis
Chemical Synthesis Overview
Definition and Goal
Chemical synthesis is the process of creating complex chemical compounds from simpler ones. Its goal is to construct molecules with precise structural configurations, which is fundamental in developing new materials, drugs, and various industrial chemicals. The success of this endeavor relies heavily on understanding the relationship between the structure of molecules and their properties.
Role in Pharmaceuticals and Materials Science
In pharmaceuticals, synthesis plays a crucial role in drug development, allowing chemists to create molecules with specific therapeutic effects. It’s essential for innovating new medicines and improving existing ones. In materials science, synthesis enables the development of new materials with unique properties, such as enhanced strength, durability, or conductivity, opening doors to advancements in technology and industry.
Linear Synthesis
Definition
Linear synthesis refers to a sequential approach to molecule construction, where the target molecule is built step by step, starting from a simple precursor and adding one piece at a time.
Key Characteristics
- Sequential nature: The process follows a straight path, with each step depending on the completion of the previous one.
- Intermediate compounds: Produces various intermediates before reaching the final product.
- Simplicity in planning: Easier to design as it follows a direct route.
Convergent Synthesis
Definition
Convergent synthesis is an approach where larger, more complex sections of the target molecule are assembled separately and then combined towards the end of the synthesis.
Key Characteristics
- Parallel construction: Different parts of the molecule are built simultaneously.
- Fewer steps: Tends to involve fewer overall steps by combining larger segments.
- Potential for higher yields: Reduced number of steps can lead to higher overall yields.
Linear Synthesis Explained
Process Details
Step-by-Step Explanation
- Selection of precursors: Identify simple molecules to start the synthesis.
- Sequential addition: Add one piece at a time to the growing chain.
- Purification of intermediates: After each step, intermediate compounds are often purified.
Advantages
- Predictability: The linear nature makes it easier to predict the outcome of each step.
- Simplicity in design: Easier to plan and execute for simpler molecules.
Challenges and Limitations
- Yield degradation: Each step may reduce the overall yield due to the potential loss of material.
- Complexity in handling intermediates: Requires managing and purifying multiple intermediates, which can be time-consuming and costly.
Applications
Examples in Drug Development
Linear synthesis is widely used in creating simple drug molecules where precision and stepwise control are crucial.
Use in Complex Molecule Construction
Despite its limitations, linear synthesis is still employed for constructing complex molecules, especially when the sequential control of stereochemistry is necessary.
Convergent Synthesis Unpacked
Process Insights
How it Works: A Closer Look
- Independent synthesis of fragments: Begin by synthesizing two or more large fragments of the target molecule separately.
- Combination of fragments: Once the fragments are ready, they are combined to form the final molecule.
Advantages
- Efficiency: By reducing the number of steps, the overall process becomes more efficient.
- Higher yields: Fewer purification steps mean less loss of material, potentially resulting in higher yields.
Challenges and Limitations
- Complexity in planning: Requires careful planning to ensure that all fragments are compatible for the final assembly.
- Dependency on fragment synthesis: The success of the final synthesis depends heavily on the successful preparation of each fragment.
Applications
Role in Efficient Molecule Assembly
Convergent synthesis is crucial for assembling complex molecules efficiently, making it a preferred method for large-scale production.
Examples in Materials Science
This approach is often used in materials science to create polymers and nanomaterials with specific properties, demonstrating its versatility beyond the pharmaceutical industry.
Comparison and Contrast
Efficiency and Yield
Comparing Synthesis Pathways
When comparing linear and convergent synthesis, the efficiency and yield of a chemical process are crucial factors. Linear synthesis, while straightforward, often involves more steps and intermediates, which can lead to a cumulative loss of yield at each stage. Convergent synthesis, by designing fewer steps and combining larger molecule fragments, typically sees less overall material loss, leading to potentially higher yields.
Impact on Yield and Purity
The purity of the final product is paramount in chemical synthesis, especially in pharmaceutical applications. In linear synthesis, the purification of each intermediate can be challenging, affecting both yield and purity. Convergent synthesis, with its reduced number of purification steps, can often produce a final product with higher purity and yield, assuming the initial fragments are themselves pure.
Time and Cost
Analysis of Time Investment
Time is a critical factor in the development and production of chemical compounds. Linear synthesis, due to its stepwise nature, can be time-consuming, especially with complex molecules requiring many intermediates. Convergent synthesis, although it requires significant upfront planning, can save time in the long run by minimizing the number of synthetic steps.
Cost Implications for Production
The cost of chemical production is directly influenced by the synthesis strategy chosen. Linear synthesis, with its extended timelines and the need for multiple purifications, can escalate production costs. Conversely, convergent synthesis, by reducing the number of steps and associated labor, can offer a more cost-effective solution for the production of complex molecules.
Scalability
Adaptability to Large-Scale Production
Scalability is a vital consideration for any synthesis strategy, especially for compounds intended for commercial production. Linear synthesis can face challenges in scalability due to the complexity of managing multiple intermediates on a large scale. Convergent synthesis, with its fewer steps and reduced complexity, is often more adaptable to large-scale production environments.
Linear vs. Convergent in Industry Settings
In industry settings, the choice between linear and convergent synthesis often depends on the specific compound being produced and the scale of production. While linear synthesis may be preferred for simpler molecules or when precise control over stereochemistry is required, convergent synthesis is frequently chosen for its efficiency and scalability in producing complex molecules.
Complexity and Versatility
Handling Complex Molecules
The complexity of the target molecule significantly influences the choice of synthesis strategy. Linear synthesis provides a level of precision and control that is beneficial for constructing highly complex molecules with multiple stereocenters. However, convergent synthesis offers a strategic advantage in managing complexity by building complex molecules from simpler, well-defined fragments.
Flexibility in Synthesis Planning
Flexibility in planning is crucial for successfully synthesizing complex chemical compounds. Convergent synthesis provides greater flexibility, allowing chemists to modify or optimize the synthesis of individual fragments before their final combination. This flexibility can be a significant advantage when unforeseen challenges arise during synthesis.
Strategic Considerations
Choosing a Synthesis Method
Factors to Consider
Selecting the most appropriate synthesis method involves several critical factors:
- Complexity of the target molecule: Determines the feasibility of a linear or convergent approach.
- Desired yield and purity: Affects the choice based on the efficiency of each method.
- Cost and time constraints: Guides the selection towards the most resource-efficient strategy.
- Scalability requirements: Influences the choice based on adaptability to large-scale production.
Case Studies
Exploring case studies of successful synthesis projects can provide valuable insights into the decision-making process. For instance, the synthesis of complex natural products often employs a convergent strategy to handle the molecule’s complexity efficiently. In contrast, simpler molecules or those requiring precise stereochemical control might be better suited to a linear approach.
Future Directions
Trends in Synthetic Chemistry
The field of synthetic chemistry is continually evolving, with new technologies and methodologies emerging that expand the possibilities of molecule construction. One notable trend is the increasing integration of computational tools to predict the most efficient synthesis routes, whether linear, convergent, or a hybrid approach.
Potential for Hybrid Approaches
Hybrid approaches that combine the best features of linear and convergent synthesis are becoming more common. Such strategies can offer enhanced efficiency, yield, and scalability, adapting dynamically to the specific challenges of each synthesis project. As research progresses, we can expect to see more innovative hybrid techniques being developed, further blurring the lines between linear and convergent synthesis strategies.
Frequently Asked Questions
What is chemical synthesis?
Chemical synthesis refers to the process of constructing complex chemical compounds from simpler ones. It serves as a foundational technique in creating various materials and compounds essential for research, pharmaceuticals, and industrial applications, enabling scientists to study and harness the properties of new substances.
Why is the choice between linear and convergent synthesis important?
Choosing between linear and convergent synthesis is crucial because it can significantly affect the efficiency, yield, and overall cost of producing complex molecules. The decision impacts the number of steps and intermediates involved, directly influencing the scalability and feasibility of the synthesis for practical applications.
How does convergent synthesis improve yield?
Convergent synthesis can improve yield by reducing the number of steps and intermediates involved in the synthesis process. By assembling larger sections of the molecule in parallel, it minimizes the opportunities for side reactions and degradation of intermediates, leading to a more efficient pathway and potentially higher yields of the final product.
Can linear and convergent synthesis be combined?
Yes, linear and convergent synthesis strategies can be combined in the synthesis of complex molecules. This hybrid approach allows chemists to leverage the advantages of both strategies, optimizing the synthesis route for efficiency, yield, and scalability. Tailoring the synthetic plan to the specific needs of the molecule can yield superior results.
Conclusion
In the realm of chemical synthesis, the distinction between linear and convergent strategies is more than a mere technicality; it represents a critical decision point that can define the success of a synthetic route. By understanding the inherent differences and strategic applications of each approach, researchers can tailor their methodologies to suit the unique demands of their target molecules, balancing efficiency, yield, and complexity.
The ongoing evolution of synthetic chemistry promises to further refine and perhaps even blur the lines between these methodologies, as new techniques and understandings emerge. Ultimately, the choice between linear and convergent synthesis is not just a matter of preference but a strategic decision that hinges on the nuanced demands of modern scientific inquiry and production.