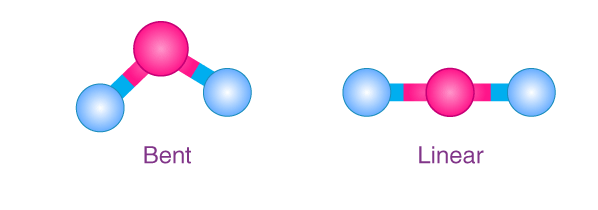
Molecular geometry is a fundamental concept in chemistry that describes the three-dimensional arrangement of atoms within a molecule. This arrangement significantly impacts the molecule’s physical and chemical properties. Two common shapes in molecular geometry are linear and bent, each presenting unique characteristics and implications in various chemical contexts.
Linear molecules are characterized by all atoms lying in a straight line, typically resulting in symmetrical shapes and properties. In contrast, bent molecules deviate from this straight arrangement, leading to a central atom bonded at an angle to adjacent atoms, which affects the molecule’s symmetry and properties. The difference between these shapes can influence molecular polarity, reactivity, and interactions with other molecules.
Understanding the distinctions between linear and bent molecules aids in predicting how a molecule behaves in different environments, from industrial applications to biological systems. These shapes determine how molecules interact with light, other chemicals, and their stability under different conditions.
Molecular Geometry Basics
Definition of Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. This configuration dictates how atoms are positioned relative to each other and profoundly influences the physical and chemical properties of the substance. Understanding molecular geometry is critical in fields such as chemistry, pharmacology, and materials science, where the shape of molecules can determine the outcomes of chemical reactions and the functionality of materials.
Factors Influencing Shape
Several key factors determine the shape of a molecule:
- Electron Configuration: The arrangement of electrons around an atom directly impacts the shape, as electrons will arrange themselves to minimize repulsion.
- Bonding Requirements: The type and number of chemical bonds (single, double, triple) an atom can form influence the spatial arrangement of atoms.
- Lone Pairs: Non-bonding electrons or lone pairs can also repel bonded pairs of electrons, altering the angle between bonds and thus the overall shape.
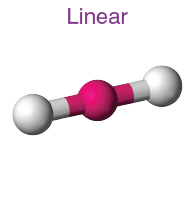
Linear Molecules
Characteristics of Linear Molecules
Linear molecules are defined by their atoms arranged in a straight line. This simplicity in structure leads to specific traits:
- Non-polarity: If all the atoms in a linear molecule are the same, the molecule is non-polar because the electrical charges are evenly distributed.
- Symmetry: Linear molecules often exhibit a high degree of symmetry, affecting their optical and electrical properties.
Common Examples
Some typical examples of linear molecules include:
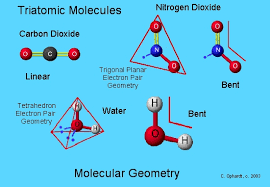
- Carbon Dioxide (CO2): A common gas where a carbon atom is double bonded to two oxygen atoms, all lying in a straight line.
- Acetylene (C2H2): An important industrial gas used for welding, consisting of two carbon atoms triple-bonded to each other with hydrogen atoms attached at each end.
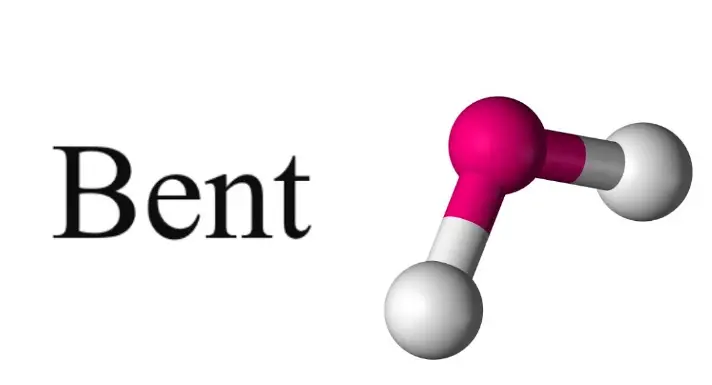
Bent Molecules
Characteristics of Bent Molecules
Unlike linear molecules, bent molecules have a central atom bonded at an angle to its surrounding atoms. This arrangement results in several distinguishing features:
- Polarity: Bent molecules are usually polar due to the uneven distribution of electrical charges caused by the bent shape.
- Reduced Symmetry: The bent configuration breaks the symmetry seen in linear molecules, affecting how they interact with light and other electromagnetic fields.
Common Examples
Familiar examples of bent molecules include:
- Water (H2O): Perhaps the most well-known bent molecule, where two hydrogen atoms form an angle with an oxygen atom at the center.
- Sulfur Dioxide (SO2): A toxic gas where a sulfur atom is bonded to two oxygen atoms in a V-shaped arrangement.
VSEPR Theory
Basics of VSEPR Theory
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a model used to predict the geometry of individual molecules based on the repulsion between sets of valence electrons surrounding an atom. The theory posits that electron pairs, whether in bonding or lone pairs, will arrange themselves as far apart as possible to minimize repulsion, thus determining the molecule’s shape.
Application to Linear and Bent Shapes
VSEPR theory explains why molecules adopt specific shapes:
- Linear: In molecules like CO2, the central carbon atom has no lone pairs and forms two double bonds with oxygen atoms, allowing for a linear shape as the pairs are as far apart as possible.
- Bent: In water (H2O), the oxygen atom has two lone pairs and two bonding pairs, causing the molecule to adopt a bent shape to minimize repulsion between these electron pairs.
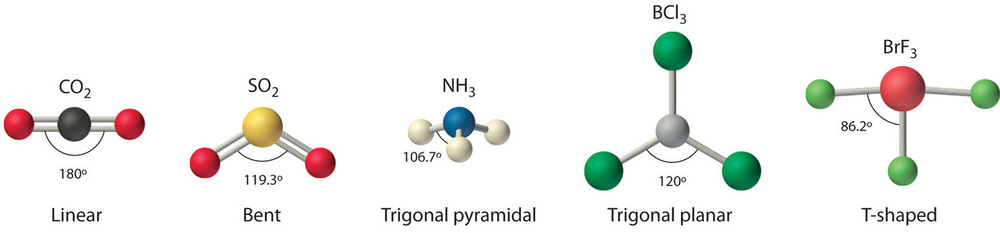
Bond Angles and Energy
Importance of Bond Angles
Bond angles play a crucial role in defining the shape and stability of molecules. These angles are the geometric parameters that directly influence the spatial arrangement of atoms in a molecule, impacting how they interact with their environment. In molecular geometry, the bond angles determine whether a molecule will be linear, bent, or adopt another shape. For example, the bond angles in water (about 104.5 degrees) make it a bent molecule, which is essential for its unique chemical properties.
Energy Implications
The energy state of a molecule is also closely linked to its bond angles. Adjustments in bond angles can lead to changes in the potential energy of a molecule, affecting its reactivity and stability. Molecules strive to adopt a geometry that minimizes their potential energy, leading to more stable configurations. This stability is vital in chemical reactions where energy efficiency can influence reaction rates and outcomes.
Physical Properties
Impact on Boiling and Melting Points
The molecular shape, influenced by bond angles, significantly affects physical properties like boiling and melting points. Linear molecules, due to their symmetrical shape, often have lower boiling and melting points compared to bent molecules, which are less symmetrical and exhibit stronger intermolecular forces. For instance, carbon dioxide (a linear molecule) sublimates at -78 degrees Celsius, whereas water (a bent molecule) has a much higher melting point of 0 degrees Celsius.
Solubility and Density Differences
Molecular geometry also determines solubility and density. Linear molecules, typically non-polar, are more likely to dissolve in non-polar solvents, whereas polar bent molecules dissolve well in polar solvents like water. Density, influenced by how tightly molecules can pack, varies with molecular shape; linear molecules tend to pack more efficiently, often resulting in higher densities.
Chemical Behavior
Reactivity Differences
The reactivity of a molecule often depends on its molecular geometry. Linear molecules, with their extended shape and even distribution of electrons, tend to react differently compared to bent molecules, which may have regions of higher electron density due to their shape. This difference is particularly noticeable in reactions involving polar and non-polar molecules.
Role in Chemical Synthesis
Molecular shape is a key factor in synthetic chemistry, where the reactivity and orientation of molecules can influence the outcome of a synthesis. For example, the bent shape of water makes it a good nucleophile in many reactions, while the linear shape of carbon dioxide makes it less reactive in similar conditions.
Spectroscopy and Analysis
IR Spectroscopy Distinctions
Infrared (IR) spectroscopy, a technique used to identify molecular vibrations, can distinguish between linear and bent molecules based on their IR spectra. Linear molecules typically show simpler spectra due to their symmetrical structure, while bent molecules display more complex patterns due to varied vibrational modes.
Use in Identifying Molecule Shapes
IR spectroscopy is not only pivotal in distinguishing between different types of molecular vibrations but also crucial in determining the shape of molecules. By analyzing the absorption peaks, scientists can deduce the presence of linear or bent geometries, aiding in the structural identification of unknown compounds.
Real-World Applications
Linear Molecules in Industry
Linear molecules have numerous applications in industry. For instance, carbon dioxide is used extensively in the food and beverage industry for carbonation of drinks. Acetylene, another linear molecule, is crucial in welding due to its ability to produce a high-temperature flame.
Bent Molecules in Nature
Bent molecules are equally significant in natural processes. Water, with its unique bent shape, is essential for life, influencing everything from cellular processes to weather patterns. Another example is sulfur dioxide, which plays a role in the formation of acid rain and is a byproduct of volcanic activity.
Frequently Asked Questions
What defines a linear molecule?
A linear molecule is defined by its atoms arranged in a straight line without any angles between them. This structure leads to unique physical properties like lower polarity and specific types of chemical reactivity.
How do bent molecules differ?
Bent molecules feature atoms arranged at an angle, not in a straight line. This shape results in higher polarity and can significantly affect how the molecule interacts with other substances and how it is used in applications like pharmaceuticals.
Why is molecular geometry important?
Molecular geometry is crucial because it determines a molecule’s physical and chemical properties. Understanding the geometry helps scientists predict molecule behavior in various reactions and applications.
What role does VSEPR theory play?
VSEPR (Valence Shell Electron Pair Repulsion) theory helps predict the shape of a molecule based on the repulsions between electron pairs surrounding an atom. This theory is fundamental in understanding why molecules adopt linear or bent shapes.
Conclusion
The exploration of linear and bent molecules provides profound insights into the behavior and characteristics of chemicals based on their molecular geometry. This knowledge is instrumental in fields ranging from material science to biochemistry, where the shape of a molecule can dictate its functionality and effectiveness.
By understanding the fundamental differences and applications of these molecular structures, scientists and engineers can design more effective drugs, better materials, and innovative solutions to chemical challenges. This understanding not only enriches academic and professional pursuits but also enhances the technological capabilities of various industries.