Catalysts are substances that speed up chemical reactions without being consumed, and they play a pivotal role in industrial and laboratory processes. Among the various catalysts used today, Lindlar and Rosenmund catalysts are particularly noteworthy due to their specific applications in organic chemistry. These catalysts facilitate different types of hydrogenation reactions, each suitable for distinct chemical tasks.
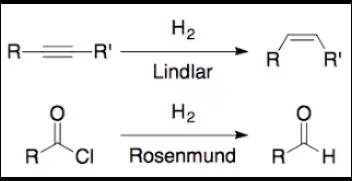
Lindlar and Rosenmund catalysts are used to control hydrogenation reactions, with the former primarily used for the partial hydrogenation of alkynes to cis-alkenes, and the latter for the hydrogenation of acid chlorides to aldehydes. This selective functionality makes them invaluable in synthetic organic chemistry where precision and efficiency are crucial.
The distinction between these catalysts lies in their composition and the specific reactions they catalyze. Lindlar’s catalyst, a palladium on calcium carbonate partially poisoned with lead, is known for its gentle action on triple bonds. Conversely, the Rosenmund catalyst, which comprises palladium deposited on barium sulfate and further poisoned with sulfur compounds, is tailored for safer aldehyde synthesis.

Lindlar Catalyst
Definition and Composition
The Lindlar catalyst is a finely tuned tool in organic chemistry, designed for specific hydrogenation reactions. This catalyst consists of palladium deposited on calcium carbonate, partially poisoned with lead acetate. This unique composition makes it highly effective for catalyzing the hydrogenation of alkynes to alkenes, specifically the cis-alkenes, without further reduction to alkanes.
Mechanism of Action
The action mechanism of the Lindlar catalyst involves the selective absorption of hydrogen and the alkyne on the palladium surface. This setup facilitates the addition of hydrogen atoms to the carbon-carbon triple bond of alkynes, converting them into double bonds (alkenes). The presence of lead acetate as a poison inhibits the complete hydrogenation to an alkane, making this process exceptionally selective for synthesizing cis-alkenes.
Common Uses in Reactions
Lindlar catalysts are prominently used in:
- Synthesis of vitamins and hormones where the cis-configuration of double bonds is crucial.
- Manufacturing of fragrances and flavor compounds, which often require specific chemical structures for their characteristic smells and tastes.
- Drug development, particularly in the formation of semi-synthetic molecules with complex, precise structures.
Advantages and Limitations
Advantages:
- High selectivity for cis-alkenes.
- Requires milder conditions compared to other hydrogenation catalysts.
Limitations:
- Poisoned catalyst can be sensitive to the reaction environment.
- Not suitable for use in reactions requiring complete hydrogenation to alkanes.
Rosenmund Catalyst
Composition Details
The Rosenmund catalyst is composed of palladium supported on barium sulfate and poisoned with sulfur compounds. This specific setup is tailored to reduce the activity of palladium, allowing for selective reduction of acid chlorides to aldehydes without over-reduction to alcohols.
Catalytic Mechanism
In the Rosenmund reduction, the catalyst’s surface interacts with the acid chloride and hydrogen gas. The sulfur poison limits the number of active sites available on the palladium, controlling the hydrogenation process to stop at the aldehyde stage. This precision prevents the formation of unwanted alcohol by-products.
Applications in Industry
Rosenmund catalysts are vital in:
- Pharmaceuticals production, where they help synthesize aldehyde intermediates crucial for various drugs.
- Fine chemicals manufacturing, including the production of fragrances and flavoring agents where aldehydes are key components.
Pros and Cons
Pros:
- Highly selective for producing aldehydes.
- Efficient under controlled conditions.
Cons:
- Catalyst deactivation due to sulfur poisoning can be an issue.
- Requires careful handling and specific reaction setups.
Comparative Analysis
Similarities Between the Two
Both Lindlar and Rosenmund catalysts share some similarities:
- Based on palladium as the active metal.
- Designed for specific, selective hydrogenation reactions.
- Require poisoning to regulate their activity.
Key Differences
While both catalysts are used for hydrogenation, their key differences lie in:
- Target reactions: Lindlar is used for alkynes to alkenes, Rosenmund for acid chlorides to aldehydes.
- Poisoning agents: Lead for Lindlar, sulfur for Rosenmund.
Reaction Specificity
The specificity of these catalysts highlights their importance in organic synthesis:
- Lindlar Catalyst: Best suited for reactions needing partial hydrogenation with a high degree of control over the product’s geometry.
- Rosenmund Catalyst: Ideal for reductions where stopping at the aldehyde stage is crucial, preventing further reduction to an alcohol.
Practical Applications
Case Studies in Synthetic Chemistry
The Lindlar and Rosenmund catalysts have been instrumental in several breakthroughs in synthetic chemistry. One notable example is the synthesis of vitamin K analogues using the Lindlar catalyst. This process involves the partial hydrogenation of a specific alkyne to a cis-alkene, crucial for the biological activity of vitamin K.
Another example is the use of the Rosenmund catalyst in developing synthetic intermediates for antidepressants. By selectively reducing acid chlorides to aldehydes, researchers can obtain compounds that are vital for the final structure of these medications.
Industrial Relevance and Examples
In the industrial sector, these catalysts have a range of applications:
- Perfume and flavor industries: The Lindlar catalyst helps in synthesizing cis-alkenes used as intermediates in fragrance compounds. Meanwhile, the Rosenmund catalyst plays a critical role in producing aldehyde-based flavorings.
- Agricultural chemicals: Specific pesticides and herbicides rely on intermediates produced through reactions facilitated by these catalysts, enhancing their effectiveness and stability.
Handling and Safety
Safety Precautions for Use
Handling these catalysts requires careful attention to safety due to their reactive nature and the presence of toxic materials like lead in the Lindlar catalyst. Essential precautions include:
- Proper ventilation: Always use these catalysts in a well-ventilated area to avoid inhaling any fumes.
- Use of personal protective equipment (PPE): Gloves, goggles, and lab coats are mandatory to protect against accidental spills and splashes.
- Training and protocols: Anyone using these catalysts should be trained in their proper handling and emergency response procedures.
Storage and Disposal Guidelines
Storing and disposing of Lindlar and Rosenmund catalysts must be done with consideration for environmental and safety impacts:
- Storage conditions: Keep the catalysts in cool, dry places and in tightly sealed containers to prevent degradation and accidental exposure.
- Disposal: Follow local regulations for the disposal of chemical waste. These catalysts, especially those containing heavy metals, may require special handling to prevent environmental contamination.
Future Prospects
Recent Advancements
Recent advancements in catalyst technology include the development of more environmentally friendly and efficient versions of the Lindlar and Rosenmund catalysts. Researchers are exploring alternatives to toxic lead-based poisons in the Lindlar catalyst and improving the sulfur poisoning technique in the Rosenmund catalyst to extend its usability and effectiveness.
Potential Developments
The future of these catalysts looks promising with ongoing research into:
- Nano-engineering: Enhancing the efficiency and selectivity of these catalysts through nano-scale modifications could lead to smaller quantities of catalysts being needed and lower costs.
- Greener alternatives: Finding safer, non-toxic alternatives to current poisons used in these catalysts is a high priority. This development would make these catalysts more suitable for a wider range of applications and reduce their environmental impact.
- Automation in synthesis: Integrating these catalysts into automated synthetic processes could improve reproducibility and scalability in pharmaceutical manufacturing and other chemical industries.
FAQs
What is Lindlar Catalyst?
Lindlar catalyst is a specially prepared catalyst consisting of palladium deposited on calcium carbonate and treated with various additives to control its reactivity. It is primarily used for the partial hydrogenation of alkynes to cis-alkenes, avoiding further reduction to alkanes.
How does Rosenmund Catalyst Work?
The Rosenmund catalyst is used for reducing acid chlorides to aldehydes without over-reduction to alcohols. This catalyst features palladium on barium sulfate, which is poisoned with sulfur compounds to finely tune its hydrogenation activity.
When to Use Lindlar vs. Rosenmund?
Choose Lindlar catalyst when you need to partially hydrogenate an alkyne to a cis-alkene. Opt for the Rosenmund catalyst when converting an acid chloride to an aldehyde is required. Each catalyst is designed for these specific reactions to provide high selectivity and yield.
Are Lindlar and Rosenmund Catalysts Reusable?
Both catalysts can be reused under certain conditions but their longevity and effectiveness can decrease over time. Proper handling and storage are essential to maintain their activity for repeated use in synthesis processes.
Conclusion
In summary, Lindlar and Rosenmund catalysts serve as essential tools in the chemist’s arsenal, each suited to specific types of hydrogenation reactions. Understanding their distinct functionalities helps in applying the correct catalyst for the desired chemical transformation.
Exploring these catalysts not only enhances efficiency in chemical synthesis but also contributes to advancements in the field of organic chemistry. As research progresses, further refinements and applications of these catalysts are likely to emerge, continuing to broaden the horizons of synthetic possibilities.