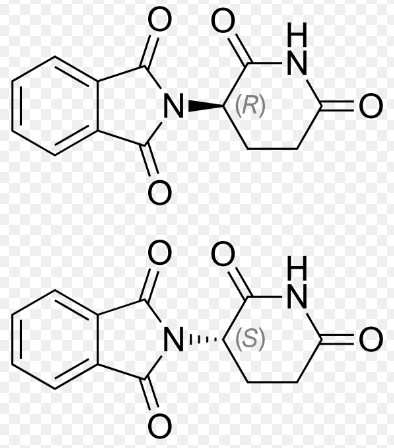
Lenalidomide and Thalidomide, two potent medications with distinct profiles, are primarily recognized for their roles in treating different medical conditions, including cancer. Both drugs share a historical lineage with profound impacts on medical science, yet they differ significantly in usage, effectiveness, and safety. The significance of these differences cannot be understated as they guide therapeutic choices and patient management.
Lenalidomide is often used in the treatment of multiple myeloma and certain myelodysplastic syndromes, characterized by its immunomodulatory effects that help control the growth and survival of cancer cells. On the other hand, Thalidomide, originally marketed as a sedative, is now used primarily to treat conditions like multiple myeloma and leprosy due to its anti-inflammatory properties. Though both are used in similar conditions, their mechanisms and side effects vary markedly.
The discussion of Lenalidomide and Thalidomide encompasses more than just their clinical applications; it involves understanding their chemical properties, medical uses, side effects, and the ethical and regulatory landscapes that shape their usage. Their development has been both celebrated and scrutinized, reflecting a complex journey from laboratory to patient care that underscores the dynamic nature of pharmaceutical development.
Historical Background
Lenalidomide Development
Origin and Medical Approval
Lenalidomide, a derivative of thalidomide, was developed with the intent to harness therapeutic benefits while minimizing harmful side effects. Approved by the FDA in 2005, Lenalidomide was a breakthrough in treating multiple myeloma, a type of cancer that affects plasma cells in the bone marrow. The drug’s approval followed significant clinical trials that showcased its efficacy in both extending survival and improving the quality of life for patients suffering from this severe condition.
Initial Uses and Clinical Significance
Initially, Lenalidomide was used to treat myelodysplastic syndromes, a group of bone marrow disorders characterized by the ineffective production of blood cells. It became particularly notable for its role in treating patients with a deletion in chromosome 5q, demonstrating a significant reduction in the need for blood transfusions. Lenalidomide’s ability to modulate the immune system and inhibit the growth of blood vessels within tumors marked it as a critical advancement in cancer therapy.
Thalidomide Development
Discovery and Early Uses
Thalidomide was first synthesized in 1957 in Germany and was introduced as a sedative and anti-nausea medication, particularly for pregnant women. It quickly became popular due to its perceived safety and efficacy. However, its widespread use came with devastating consequences as it led to severe birth defects when taken by pregnant women, an event that unfolded one of the biggest pharmaceutical tragedies in history.
Historical Controversies and Impact
The thalidomide disaster led to stricter drug regulations and a reevaluation of drug approval processes globally, emphasizing the importance of rigorous clinical testing and post-marketing surveillance. Despite its notorious past, research in the late 1990s revived thalidomide for new uses, such as cancer treatment, particularly in multiple myeloma, thanks to its anti-inflammatory and antiangiogenic properties.
Chemical Properties
Lenalidomide Structure
Molecular Composition
Lenalidomide is chemically similar to thalidomide but has modifications that enhance its efficacy and reduce toxicity. Its structure allows it to bind more effectively to target proteins involved in the immune response and the environment of cancer cells.
Mechanism of Action
The action mechanism of Lenalidomide involves the modulation of the body’s immune system, boosting the natural defense against cancerous cells and interfering with the microenvironment that supports cancer growth. This dual action makes it particularly effective in treating blood cancers.
Thalidomide Structure
Chemical Makeup
Thalidomide features a simple yet effective structure that enables it to modulate immune system responses and inhibit angiogenesis, the process by which new blood vessels form, which is crucial in the spread and growth of tumors.
Action Mechanism
The primary mechanism of Thalidomide involves the prevention of new blood vessel formation, a vital process for tumor growth and survival. Additionally, Thalidomide modulates various cellular signaling pathways involved in inflammation and immunity, making it useful in treating diseases characterized by excessive inflammatory responses.
Medical Uses
Current Applications of Lenalidomide
Cancer Treatments
Lenalidomide is most notably used in the treatment of multiple myeloma and certain types of lymphoma. Its effectiveness in these cancers is due to its ability to stimulate the immune system and inhibit the vascularization of tumors, starving the cancer cells of necessary nutrients.
Other Medical Conditions
Beyond cancer, Lenalidomide has shown potential in treating autoimmune conditions due to its immune-modulating capabilities. Trials continue to explore its efficacy in diseases like lupus and rheumatoid arthritis.
Current Applications of Thalidomide
Prescribed Conditions
Today, Thalidomide is used primarily to treat conditions like erythema nodosum leprosum, a severe form of leprosy, and in cases of multiple myeloma, where it is used in combination with other drugs to enhance therapeutic outcomes.
Recent Medical Research
Recent studies have explored Thalidomide’s potential in treating other conditions, including other autoimmune disorders and inflammatory diseases, due to its properties of reducing inflammation and modulating the immune system. The drug’s ability to interfere with tumor necrosis factor (TNF) and inhibit angiogenesis opens new avenues for its application in medical science.
Side Effects and Safety
Risks of Lenalidomide
Common and Severe Side Effects
Lenalidomide, though effective, carries a risk profile that demands careful consideration. Common side effects include fatigue, diarrhea, and itchiness. More severe risks involve neutropenia (low white blood cell count) and thrombocytopenia (low platelet count), which increase the risk of infection and bleeding, respectively. Notably, Lenalidomide has been linked to an increased risk of developing second primary malignancies, a concern that requires vigilant monitoring.
Safety Measures and Monitoring
To mitigate these risks, patients on Lenalidomide undergo regular blood tests to monitor their complete blood count (CBC) and kidney function. Additionally, due to the risk of birth defects, strict guidelines are in place for women of childbearing age, including mandatory pregnancy tests and contraception advice.
Risks of Thalidomide
Known Adverse Effects
Thalidomide’s side effects are well-documented and include peripheral neuropathy, drowsiness, and constipation. Its most notorious risk, however, remains teratogenicity — the potential to cause severe birth defects, which historically led to its initial withdrawal from the market.
Regulatory Safeguards
Given its history, Thalidomide is now subject to stringent regulatory controls under programs like the Thalidomide REMS program in the United States, which ensures that the drug is prescribed and dispensed safely to prevent pregnancy exposures.
Legal and Ethical Considerations
Regulatory Status of Lenalidomide
Approvals and Restrictions
Lenalidomide is approved in multiple countries but comes with tight regulatory oversight due to its potential risks. In the United States, it is classified under a Risk Evaluation and Mitigation Strategy (REMS) program to prevent unintended exposure during pregnancy.
Ethical Considerations in Use
The use of Lenalidomide raises ethical questions regarding patient education about its risks and the ongoing monitoring required to use the drug safely. Ensuring that patients understand and consent to these risks is paramount.
Regulatory Status of Thalidomide
Current Legal Status Worldwide
Thalidomide is approved for use but is heavily regulated across the globe due to its teratogenic effects. Its use is restricted to certain conditions and requires explicit warnings about its risks.
Ethical Debates and Usage Guidelines
The ethical debates around Thalidomide continue, particularly concerning its use in women of reproductive age and the strict guidelines that govern its prescription and distribution.
Comparative Analysis
Efficacy Comparison
Comparative Studies and Results
Studies comparing Lenalidomide and Thalidomide show that while both are effective for multiple myeloma, Lenalidomide often offers a better side effect profile and longer progression-free survival times for patients.
Analysis of Effectiveness in Different Conditions
While both drugs are used in similar conditions, Lenalidomide is generally preferred for myeloma due to less severe side effects and more potent immune-modulating effects.
Cost and Accessibility
Pricing Strategies
Lenalidomide is typically more expensive than Thalidomide, reflecting its newer patent status and broader efficacy. However, this cost can be a barrier for many patients.
Availability in Different Regions
Both medications are available globally but are subject to varying degrees of regulatory control, affecting their availability and use in different healthcare systems.
Future Prospects
Research Trends: Lenalidomide
Ongoing Trials
Ongoing clinical trials are exploring new uses for Lenalidomide in other types of cancer and diseases involving immune dysregulation.
Potential Future Applications
Future applications may expand to autoimmune diseases and more widespread cancer treatment protocols, potentially increasing its therapeutic footprint.
Research Trends: Thalidomide
Emerging Studies
New research into Thalidomide is examining its potential anti-inflammatory effects in other diseases beyond leprosy and multiple myeloma.
Prospective Medical Uses
Thalidomide may see renewed interest in fields such as neurodegenerative diseases and complex inflammatory syndromes, where modulation of the immune response and angiogenesis could prove beneficial.
Frequently Asked Questions
What is Lenalidomide used for?
Lenalidomide is primarily prescribed for treating multiple myeloma and certain myelodysplastic syndromes. It enhances the immune system’s ability to fight cancer and can also reduce blood vessel growth in tumors, which helps in controlling cancer progression.
How does Thalidomide work?
Thalidomide works by modulating the immune system and reducing inflammation. Its mechanism involves inhibiting the growth of new blood vessels, a process known as angiogenesis, which is crucial in the development and progression of cancers and inflammatory conditions.
Are Lenalidomide and Thalidomide the same?
No, Lenalidomide and Thalidomide are not the same. While both drugs are used to treat similar conditions like multiple myeloma, Lenalidomide is generally considered safer and more effective due to its refined mechanism that targets cancer cells with fewer side effects compared to Thalidomide.
What are the main side effects of Thalidomide?
The main side effects of Thalidomide include peripheral neuropathy (nerve damage), constipation, rash, fatigue, and potential severe birth defects if taken during pregnancy. Its use is heavily regulated and monitored to prevent these risks.
Can Lenalidomide cure cancer?
Lenalidomide is not a cure for cancer, but it is an effective treatment that can significantly extend survival and improve quality of life in patients with multiple myeloma and other related conditions. It is often used as part of a combination therapy regimen to maximize its efficacy.
Conclusion
The journey of Lenalidomide and Thalidomide through the pharmaceutical landscape is a testament to how medical science evolves and adapts in response to both therapeutic needs and historical lessons. Each drug carries a distinct profile that suits different therapeutic niches, backed by ongoing research that continues to refine their applications and safety measures.
Understanding their differences is crucial for clinicians and patients alike, as it aids in making informed treatment decisions that are tailored to individual health needs and conditions. As research progresses, the roles of Lenalidomide and Thalidomide in medicine are likely to expand, bringing more nuanced understanding and better patient outcomes.