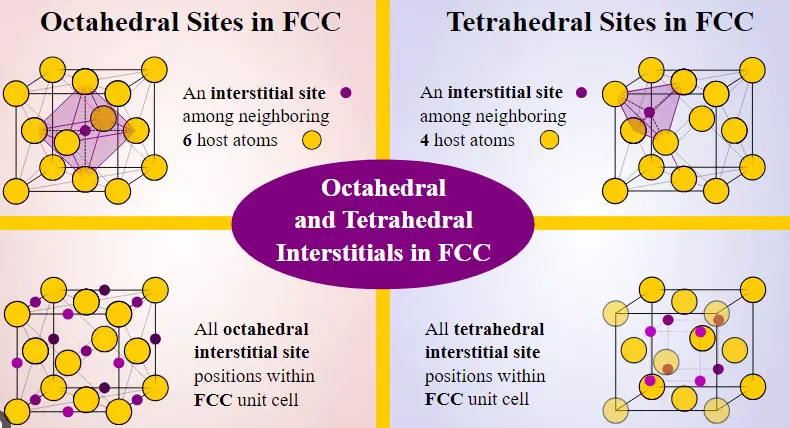
The arrangement of atoms in a crystalline solid can determine its properties, making it important to understand the difference between lattice sites and interstitial sites. In this blog, we will discuss what lattice sites and interstitial sites are, how they are different from each other, and how this difference affects the properties of the solid.
Characteristics of lattice sites
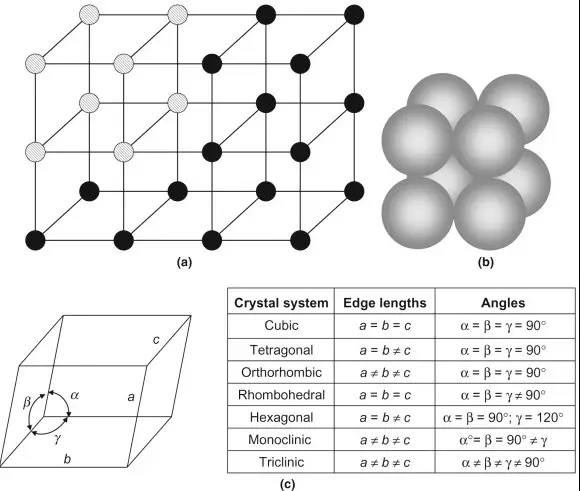
When it comes to understanding the characteristics of lattice sites, it is important to understand the difference between lattice sites and interstitial sites. A lattice site is a location in a crystal structure that is occupied by an atom or a molecule, and is usually surrounded by other atoms or molecules in a regular pattern.
On the other hand, an interstitial site is a location in a crystal structure that is not occupied by an atom or a molecule, and is usually found between the lattice sites. The main difference between them is that lattice sites are more structured and organized, while interstitial sites are more random and chaotic. Both have different effects on the physical and chemical properties of a material.
Lattice sites are responsible for the structural integrity of a material, while interstitial sites can contribute to the material’s electrical and magnetic properties.
Characteristics of interstitial sites
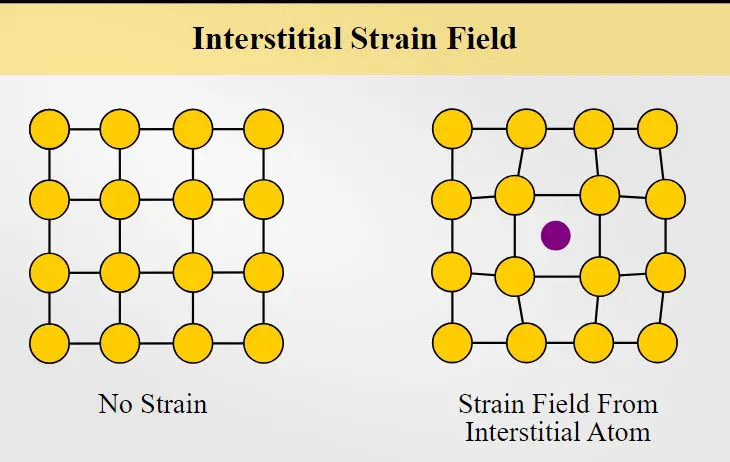
Interstitial sites refer to locations between lattice sites in a crystalline solid. While there is a clear difference between lattice sites and interstitial sites, there are a few characteristics that they share.
Both lattice sites and interstitial sites are found in regular, repeating patterns in a crystalline solid, but the differences between them are in the amount of space they occupy and the type of atoms that occupy them. Lattice sites are typically occupied by atoms of a specific type, while interstitial sites can be occupied by different types of atoms or molecules. Interstitial sites are also much smaller than lattice sites, offering less of a structural support and stability.
Despite these differences, both lattice sites and interstitial sites are important in providing a structural framework for a crystalline solid.
Comparison of lattice and interstitial sites
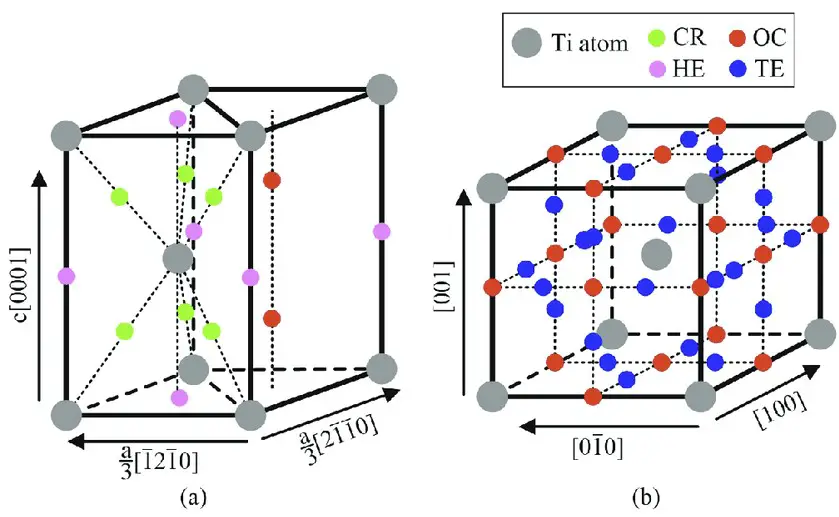
The difference between lattice sites and interstitial sites can be easily seen when looking at the structure of a material. Lattice sites are the voids between atoms that form a crystalline structure, whereas interstitial sites are sites located in the interstices between the lattice sites. Lattice sites are typically found in substances with a regular arrangement of atoms or molecules, such as metals and crystals.
Lattice sites are typically found in substances with a regular arrangement of atoms or molecules, such as metals and crystals. Interstitial sites, on the other hand, are usually found in more disordered materials, such as polymers and ceramics. In terms of properties, interstitial sites have a much higher energy content than lattice sites due to the presence of extra atoms or molecules occupying the site.
This higher energy content results in higher reactivity, which can be seen in the increased electrical or heat conductivity of interstitial materials. Lattice sites, on the other hand, have a lower energy content, and thus, lower reactivity.
This can be seen in the lower electrical and heat conductivity of lattice materials. Overall, the main difference between lattice and interstitial sites is the arrangement of atoms or molecules and the associated energy content. Lattice sites are found in regular structures and have lower energy content, while interstitial sites are found in disordered structures and have higher energy content.
Advantages of lattice sites
Lattice sites and interstitial sites are two different types of sites within a crystal lattice. While they may look similar, they have distinct advantages and disadvantages.
Lattice sites offer higher packing densities, allowing for more efficient packing of atoms and molecules. This makes them optimal for energy storage and other applications.
On the other hand, interstitial sites have a larger surface area, allowing for faster diffusion of molecules and ions, making them ideal for a wide range of applications. Both types of sites are important for the functionality of crystals, and each type has its own advantages and disadvantages.
Advantages of interstitial sites
Interstitial sites are a type of crystallographic site in a lattice that lies between the lattice points of the crystal structure. Unlike lattice sites, interstitial sites are not located at the exact points of the lattice, but rather in the spaces between them. This unique positioning gives interstitial sites a number of advantages over lattice sites.
For example, since interstitial sites are not restricted to the exact lattice points, they have greater flexibility in accommodating atoms or molecules of different sizes. This makes interstitial sites particularly suitable for hosting specific molecules or ions which may be too large to fit into lattice sites.
Additionally, since interstitial sites exist within the lattice, they are more easily accessible for binding and reactions. This makes it possible to form different types of bonds between ions or molecules that would not be possible at lattice sites. Finally, interstitial sites are able to store energy, making them useful for storing energy in materials.
Finally, interstitial sites are able to store energy, making them useful for storing energy in materials. All of these advantages make interstitial sites an attractive option for materials scientists working in the field of crystal engineering.
Conclusion
In conclusion, the main difference between a lattice site and an interstitial site is that a lattice site is located within a lattice structure, while an interstitial site is located between lattice points. Lattice sites are also characterized by having a specific crystal structure, while interstitial sites are not necessarily organized in a specific manner. Additionally, lattice sites are usually found in a variety of metals, while interstitial sites are usually found in a single type of metal.
Finally, the formation of lattice sites requires significantly more energy than the formation of interstitial sites.