Organic chemistry introduces a myriad of compounds with unique structures and applications, including lactide and lactone. These compounds, often overlooked, play crucial roles in various industrial and pharmaceutical contexts. Their distinct chemical identities and uses make them fascinating subjects of study. Lactide and lactone, while sounding similar, are different in terms of their chemical makeup and applications.
Lactide is a cyclic diester of lactic acid and serves as a monomer in the production of polylactic acid, a biodegradable polymer. Lactone, on the other hand, refers to any of a broad class of cyclic esters formed from hydroxy acids. Both compounds are integral to eco-friendly materials and have unique pathways and implications in synthesis and degradation.
Exploring the differences between lactide and lactone reveals their distinct formation processes, physical and chemical properties, and the significant roles they play in environmental sustainability. Their relevance spans from biodegradable plastics in medical devices to eco-friendly packaging solutions, highlighting their importance in current and future technological and environmental advancements.
Basic Definitions
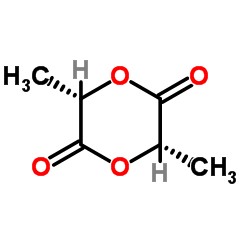
What is Lactide?
Lactide is a cyclic diester derived from lactic acid. This compound is primarily known for its role as a monomer in the production of polylactic acid (PLA), a type of biodegradable plastic that has gained attention for its environmental benefits. Lactide is not just a chemical entity but a pivotal component in sustainable materials development.
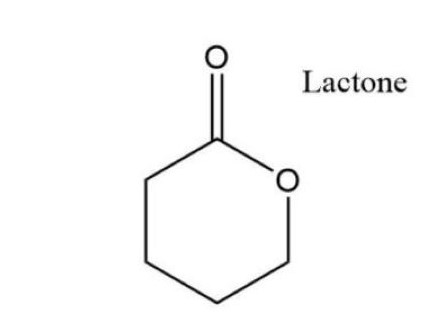
What is Lactone?
A lactone is a compound formed by the cyclic esterification of a hydroxy acid. Unlike lactide, lactones encompass a broad class of chemicals used across various sectors. These organic compounds are crucial in pharmaceuticals, aromatics, and as intermediates in numerous chemical reactions.
Chemical Structures
Structure of Lactide
The chemical structure of lactide consists of a ring formed by the ester linkage of two lactic acid molecules. This ring structure is significant because it provides the necessary reactivity for polymerization. Lactide typically appears as a crystalline solid and is stable under normal conditions.
Structure of Lactone
Lactones are characterized by their internal ester bonds, which form a ring. The size of the ring can vary, influencing the compound’s reactivity and physical properties. Lactones are known for their versatility in synthesis, thanks to the variability in ring size and substituent groups.
Formation Processes
How Lactide is Formed
The formation of lactide involves several key steps:
- Fermentation of sugars produces lactic acid.
- Lactic acid molecules undergo condensation, a dehydration reaction, leading to the formation of lactide.
This process is crucial for producing lactide in a high yield and purity, essential for high-quality PLA production.
How Lactone is Formed
Lactones form through a somewhat different process:
- Hydroxy acids oxidize, and their carboxyl group reacts with a hydroxyl group on the same molecule.
- This reaction leads to the formation of a cyclic ester, or lactone.
The process can occur naturally in fatty acids or synthetically in a laboratory setting.
Key Properties
Physical Characteristics of Lactide
Lactide is known for its:
- High melting point and glass transition temperature, making it suitable for thermal processing.
- Optical clarity in its polymer form, which is advantageous for packaging applications.
Physical Characteristics of Lactone
Lactones vary in physical properties based on their structure, but generally, they are:
- Low-melting solids or liquids at room temperature.
- Known for their distinctive odors, which make them valuable in flavor and fragrance industries.
Chemical Properties Comparison
Comparing lactide and lactone:
- Lactide is more rigid, owing to its cyclic diester structure, which makes it suitable for sturdy biodegradable plastics.
- Lactones, with their varied ring sizes and substituents, display a range of reactivities, making them versatile in chemical synthesis.
Uses and Applications
Lactide in Industry
Lactide’s primary use is in the production of PLA, which serves multiple industries:
- Biomedical (for sutures, implants)
- Packaging (biodegradable containers)
- Agriculture (mulch films, plant pots)
The demand for PLA emphasizes the importance of lactide in contributing to eco-friendly solutions.
Lactone in Industry
Lactones find their applications in diverse fields:
- Flavor and fragrance industries, where their unique smells and tastes enhance products.
- Pharmaceuticals, where some lactones function as active pharmaceutical ingredients.
Environmental Impact
Biodegradability of Lactide
Lactide-based polymers, particularly polylactic acid (PLA), are celebrated for their biodegradability. This attribute is critical in an era where environmental sustainability is a major concern. Here’s how PLA’s biodegradability stands out:
- Decomposes under industrial composting conditions: PLA can break down into water and carbon dioxide within a few months in industrial composting facilities, which maintain high temperatures and controlled humidity.
- Home composting: While slower, PLA can also degrade in backyard compost settings, albeit over a longer period, depending on environmental conditions.
This biodegradability is a significant advantage, reducing waste in landfills and lessening environmental footprint.
Biodegradability of Lactone
Lactones, depending on their chemical structure, also show varying degrees of biodegradability. Smaller lactones tend to break down more quickly than their larger counterparts due to their simpler structures and higher solubility in water. This property makes lactones particularly useful in applications where quick degradation is beneficial, such as in fragrance release in consumer products.
Economic Aspects
Market Trends for Lactide
The global market for lactide is on the rise, driven by increasing demand for sustainable and biodegradable materials. Key points include:
- Growth in the bioplastics sector: As industries and consumers push for greener alternatives, the demand for PLA, and thus lactide, continues to grow.
- Legislative impact: Government policies favoring environmentally friendly materials in packaging, agriculture, and healthcare are boosting the market for lactide-based products.
This growth is not only beneficial for the environment but also for economies focusing on green technology.
Market Trends for Lactone
Lactone enjoys a robust market presence across various industries. The trends affecting this market include:
- Increased use in pharmaceuticals and aromatics: The need for high-purity lactones in drug synthesis and food flavorings is expanding.
- Innovation in applications: New uses for lactones in biodegradable products and high-performance materials are being developed, which broadens their market potential.
The economic implications of lactone usage are extensive, influencing sectors from luxury goods to essential services.
Future Prospects
Research Directions for Lactide
Future research on lactide is focused on enhancing its properties and applications:
- Improving biodegradability: Scientists are working on making PLA degrade more efficiently in less controlled environments.
- Expanding usage: Research into using PLA in new areas such as automotive and electronics is ongoing, which could open new markets for lactide-based products.
These research initiatives promise to make lactide even more integral to sustainable development.
Research Directions for Lactone
The future of lactone research appears promising with several key areas of focus:
- Enhanced synthesis methods: Developing more efficient, less energy-intensive methods of synthesizing lactones is a priority.
- Broader biodegradability applications: Researchers are exploring ways to use lactones in more biodegradable applications, potentially replacing more persistent chemicals in many formulations.
Frequently Asked Questions
What is Lactide?
Lactide, specifically a cyclic diester of lactic acid, is primarily used as a precursor for polylactic acid, a biodegradable polymer. It is synthesized through the condensation of lactic acid molecules, which results in its distinct ring structure that is essential for polymerization.
What is Lactone?
Lactone encompasses a group of compounds characterized by a cyclic ester configuration formed from the oxidation of hydroxy acids. These compounds are widely used in pharmaceuticals, flavorings, and as precursors in various chemical syntheses due to their stability and reactivity.
How are Lactide and Lactone different?
The primary difference between lactide and lactone lies in their chemical structures and formation processes. Lactide forms through the dimerization of lactic acid and is specific to polylactic acid production, whereas lactones can be formed from various hydroxy acids and have broader applications across different industries.
What are the uses of Lactide and Lactone?
Lactide is extensively used in the production of bioplastics, notably in the medical field for biodegradable sutures and implants. Lactone finds its applications in the creation of flavoring agents, pharmaceuticals, and fine chemicals, demonstrating its versatility in different sectors.
Why are Lactide and Lactone important for sustainability?
Both lactide and lactone contribute significantly to sustainability efforts due to their biodegradability and roles in producing eco-friendly products. Lactide-derived polylactic acid helps reduce plastic waste, while lactones’ diverse applications include biodegradable products that diminish environmental impact.
Conclusion
Lactide and lactone, though often confused, are distinctly different in their chemical nature and applications. Their study not only contributes to a better understanding of organic chemical processes but also underpins important industrial applications that align with sustainable development goals. The diverse uses and potential of both compounds underscore their importance in ongoing environmental and technological solutions.
As research continues to evolve, the roles of lactide and lactone are set to become even more integral to advancements in biodegradable materials and green chemistry. Their continued development and application hold promise for significant contributions to environmental sustainability, making their study essential for future innovations.