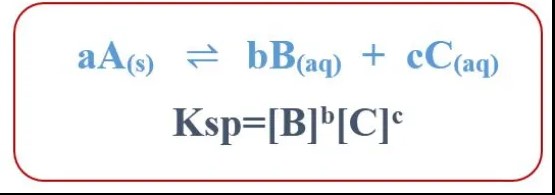Chemical equilibria form the cornerstone of many processes both in nature and in industrial applications, highlighting the delicate balance between reactants and products in chemical reactions. At the heart of understanding these equilibria lie two pivotal concepts: the Solubility Product Constant (Ksp) and the Equilibrium Constant (Keq). These terms, often encountered in chemistry, are crucial for predicting the extent to which a reaction will proceed under given conditions.
Ksp and Keq are fundamental in determining the solubility of compounds and the direction of chemical reactions, respectively. Ksp is used to predict the solubility of ionic compounds in water, indicating the maximum amount of solute that can dissolve to form a saturated solution. Keq, on the other hand, gives insight into the ratio of the concentrations of products to reactants at equilibrium, offering a glimpse into the likelihood of a reaction’s progression or reversal.
Understanding the nuances between Ksp and Keq unravels the complex interplay of chemical substances in solution, offering a window into predicting reaction outcomes. This distinction not only aids in the academic pursuit of chemistry but also has practical applications across various industries, from pharmaceuticals to environmental science, where the principles of solubility and reaction dynamics are pivotal.
Ksp Explained
Definition
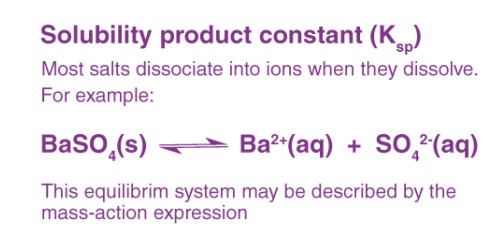
Solubility Product Constant (Ksp) is a term in chemistry that quantifies the solubility of ionic compounds in a solvent. It reflects the maximum concentration of an ionic compound that can dissolve in water to form a saturated solution. Essentially, Ksp provides a numeric value that helps predict how much of a substance can dissolve in water at a given temperature.
Role in Chemistry
The role of Ksp in chemistry is significant, especially when discussing the solubility of compounds. Understanding Ksp is crucial for:
- Predicting whether a precipitate will form in a solution.
- Determining the solubility of various salts in water.
- Facilitating the separation of ions in analytical chemistry.
Calculating Ksp
To calculate Ksp, you need to know the formula of the ionic compound and its dissociation in water. The steps include:
- Write the dissociation equation of the compound in water.
- Express the Ksp expression based on the stoichiometry of the reaction.
- Use the concentrations of the ions at equilibrium to calculate the Ksp value.
For example, for a compound AB that dissociates into A+ and B- in water, the Ksp can be expressed as:
���=[�+][�−]Ksp=[A+][B−]
Factors Affecting Ksp
Two main factors affect the Ksp value of a substance: temperature and ionic strength.
- Temperature: Solubility patterns vary with temperature. For most salts, solubility increases with an increase in temperature, thus affecting Ksp.
- Ionic Strength: The presence of other ions in the solution can affect the solubility of a compound, altering its Ksp.
Keq Explained
Definition
The Equilibrium Constant (Keq) is a fundamental concept in chemistry representing the ratio of product concentrations to reactant concentrations at equilibrium, each raised to the power of their coefficients in the balanced chemical equation. Keq offers a glimpse into the extent a reaction will proceed to reach equilibrium.
Role in Chemistry
Keq is indispensable in chemistry, serving roles such as:
- Predicting the direction of a chemical reaction.
- Calculating the concentrations of reactants and products at equilibrium.
- Understanding how changes in conditions affect chemical equilibria.
Calculating Keq
Calculating Keq involves using the concentrations of the reactants and products at equilibrium. The steps include:
- Write the balanced chemical equation for the reaction.
- Use the equilibrium concentrations of reactants and products to form the Keq expression.
- Calculate Keq using the expression.
For a reaction where ��+��→��+��aA+bB→cC+dD, Keq is expressed as:
���=[�]�[�]�[�]�[�]�Keq=[A]a[B]b[C]c[D]d
Factors Affecting Keq
Keq can be influenced by various factors, such as temperature, concentration, and pressure:
- Temperature: A change in temperature can shift the equilibrium, thus changing Keq.
- Concentration: While Keq itself remains constant, changing concentrations of reactants or products can shift the equilibrium position.
- Pressure: For reactions involving gases, changing the pressure can affect the equilibrium position and thus the observed concentrations of reactants and products.
Ksp vs Keq: Core Differences
Nature of Equilibria
- Ksp is specific to solubility equilibria, focusing on the solubility of ionic compounds in water.
- Keq applies to general chemical reactions, offering a broader view of chemical equilibria.
Mathematical Representation
- The formulas for Ksp and Keq differ, with Ksp being specific to the dissolution of ionic compounds and Keq encompassing a wide range of chemical reactions.
Applications in Chemistry
- Ksp is primarily used in contexts related to solubility, such as in predicting the formation of precipitates.
- Keq finds applications in predicting the direction and extent of chemical reactions, crucial in synthesis and reaction design.
Influence of External Factors
Both Ksp and Keq are affected by temperature, but in different ways:
- For Ksp, an increase in temperature generally increases solubility (with exceptions).
- For Keq, temperature changes can either favor the forward or reverse reaction, depending on the reaction enthalpy.
Moreover, ionic strength significantly influences Ksp by altering the activity coefficients of the ions in solution. Conversely, changes in concentration and pressure are more pertinent to Keq, as they can shift the equilibrium position without altering the Keq value itself.
Impact on Chemical Studies
Predicting Reaction Direction
The ability to predict the direction in which a chemical reaction will proceed is foundational in chemistry. This is where Keq and the Reaction Quotient (Q) come into play. Keq provides the equilibrium position of a reaction, indicating whether the products or reactants are favored under standard conditions. Q, on the other hand, is calculated in the same way as Keq but uses the current concentrations of reactants and products, not necessarily at equilibrium.
- When Q < Keq, the reaction tends to move forward, producing more products.
- When Q > Keq, the reaction is likely to reverse, forming more reactants.
- When Q = Keq, the system is at equilibrium, with no net change in the concentration of reactants or products.
Understanding the relationship between Q and Keq/Ksp allows chemists to manipulate conditions to drive reactions in a desired direction, crucial for synthesis and analysis.
Role of Q vs. Keq/Ksp
The role of Q in comparison to Keq and Ksp underscores its utility in real-time reaction monitoring. It serves as a predictive tool for determining shifts in reaction conditions, offering insights into how changes in concentration, temperature, or pressure can influence the reaction pathway. This comparison is vital in industrial processes where maintaining reaction efficiency and yield is paramount.
Industrial Applications
From Pharmaceuticals to Mining
The concepts of Keq and Ksp are not confined to academic settings; they have profound implications across various industries.
Pharmaceuticals
In pharmaceuticals, controlling solubility is essential for drug formulation and bioavailability. Manipulating Ksp allows for the optimization of drug solubility, ensuring that medications are absorbed efficiently by the body. Additionally, understanding reaction equilibria (Keq) is crucial in synthesizing new drugs, where yield and purity can significantly impact therapeutic efficacy.
Mining
In mining, Ksp principles are applied in the extraction and purification of metals from ores. Processes such as leaching rely on solubility equilibria to separate valuable metals from other components. The control of pH, temperature, and other factors allows for the selective precipitation of specific metals, enhancing recovery rates.
Academic Relevance
Teaching and Research Implications
Keq and Ksp concepts are fundamental in teaching chemistry, providing students with a comprehensive understanding of reaction dynamics and solubility principles. These concepts foster critical thinking and analytical skills, enabling students to predict and rationalize the behavior of chemical systems.
In research, Keq and Ksp are invaluable for developing new materials, studying environmental processes, and investigating biochemical pathways. They offer a quantitative framework for exploring the theoretical and practical aspects of chemistry, driving innovation and discovery.
Optimization Techniques
Enhancing Solubility
Manipulating the Ksp of a compound is a strategy often employed to enhance its solubility, especially in pharmaceuticals and materials science. Techniques include:
- pH Adjustment: Many compounds have pH-dependent solubility, where altering the pH can increase solubility.
- Use of Complexing Agents: Adding agents that form soluble complexes with the ion of interest can effectively increase its solubility.
- Temperature Control: As the solubility of most solids in liquids increases with temperature, adjusting the temperature can be a simple way to enhance solubility.
Manipulating Ksp for Better Outcomes
To manipulate Ksp effectively:
- Identify the solubility-limiting factors for the compound of interest.
- Select an appropriate method (e.g., pH adjustment, temperature control) based on the compound’s properties and the desired outcome.
- Apply the method carefully, monitoring changes in solubility and adjusting conditions as needed.
Driving Reactions
Altering conditions to favor Keq involves strategies that shift the equilibrium position to produce more desired products. Some approaches include:
- Changing Concentrations: Adding more reactants or removing products as they form can drive a reaction forward.
- Temperature Adjustment: For endothermic reactions, increasing the temperature shifts the equilibrium to the right, producing more products. The opposite applies for exothermic reactions.
- Pressure Modification: In reactions involving gases, increasing the pressure shifts the equilibrium towards the side with fewer moles of gas.
Altering Conditions to Favor Keq
To effectively drive reactions by altering Keq:
- Understand the reaction dynamics, including whether it’s endothermic or exothermic.
- Identify the most effective lever (temperature, concentration, or pressure) to manipulate for your specific reaction.
- Implement changes gradually, monitoring the reaction progress to avoid overshooting the desired equilibrium position.

Frequently Asked Questions
What is Ksp in chemistry?
Ksp, or the Solubility Product Constant, is a measure used in chemistry to denote the maximum amount of solute that can dissolve in a solvent to form a saturated solution at a specific temperature. It is particularly useful for predicting the solubility of ionic compounds in water, playing a critical role in understanding the formation of precipitates and the conditions under which a solution remains unsaturated, saturated, or supersaturated.
How does temperature affect Keq?
Temperature plays a significant role in influencing the Equilibrium Constant (Keq) for a chemical reaction. An increase in temperature generally favors the endothermic direction of a reversible reaction, thereby increasing Keq, while a decrease in temperature favors the exothermic direction, reducing Keq. This is because altering the temperature changes the reaction’s energy landscape, directly impacting the ratio of products to reactants at equilibrium.
Can Keq predict reaction speed?
Keq provides insight into the position of equilibrium and the extent to which a reaction will proceed but does not directly indicate the speed at which the reaction will reach equilibrium. It tells us about the final ratio of products to reactants but not how quickly this state will be achieved. The reaction kinetics, which involve rates of reaction and are determined by factors such as concentration, temperature, and the presence of catalysts, are responsible for dictating the speed of a reaction.
What happens when Ksp equals the product concentration?
When the product of the ion concentrations in a solution equals the Ksp value for a dissolved ionic compound, the solution is at saturation point, meaning it holds as much solute as it can without forming a precipitate. In this state, the solution is in dynamic equilibrium, with the rate of dissolution equaling the rate of precipitation. Adding more solute or changing conditions to increase the product of ion concentrations above the Ksp value will lead to precipitation.
Conclusion
Grasping the concepts of Ksp and Keq is more than an academic endeavor; it’s a gateway to understanding the dynamics of chemical reactions in the world around us. These constants offer profound insights into the behavior of substances in various environments, predicting how compounds dissolve, react, and reach equilibrium. This understanding is not only pivotal for students and researchers in chemistry but also has far-reaching implications across multiple fields where chemical reactions play a crucial role.
By demystifying the differences and applications of Ksp and Keq, we equip ourselves with the knowledge to innovate, troubleshoot, and apply chemistry in real-world scenarios more effectively. As we continue to explore the intricate dance of molecules and ions, these constants serve as fundamental tools in our quest to unravel the mysteries of the chemical world, underscoring the beauty and complexity of nature’s chemical processes.