Energy plays a pivotal role in the universe, dictating the movement of particles, the progress of reactions, and the functionality of systems both living and inanimate. At the core of these vast and varied phenomena lie two crucial concepts: kinetic energy and activation energy. While both are fundamental to the understanding of physics and chemistry, they serve distinct roles in the orchestration of matter and its behavior.
Kinetic energy is the energy an object possesses due to its motion, quantifiable by its mass and the square of its velocity. Activation energy, on the other hand, is the minimum energy required to initiate a chemical reaction. It’s the barrier that reactants must overcome to transform into products.
In the vast tapestry of science, these energies highlight the dynamism of molecules, the feasibility of reactions, and the perpetual motion within the physical world. Their understanding not only illuminates the mechanics of the microscopic but also unlocks the potential for innovation and efficiency in various fields, from engineering to biochemistry.
Kinetic Energy Explained
Definition and Formula
At its core, kinetic energy is the energy that an object possesses due to its motion. It’s a fundamental concept in physics that explains much about how and why objects move. The formula to calculate kinetic energy (KE) is quite straightforward:
��=12��2KE=21mv2
where �m represents the mass of the object and �v its velocity. This equation reveals that the kinetic energy of an object is directly proportional to its mass and the square of its velocity. In simpler terms, heavier objects moving faster have more kinetic energy.
Types and Examples
Kinetic energy can be classified into two main types: translational and rotational. Translational kinetic energy is the energy due to motion in a straight line, whereas rotational kinetic energy is due to an object’s rotation.
- Translational Kinetic Energy: For instance, a car speeding down a highway possesses translational kinetic energy because it moves from one place to another.
- Rotational Kinetic Energy: A classic example would be the Earth rotating on its axis, possessing rotational kinetic energy.
Role in Everyday Phenomena
Kinetic energy plays a crucial role in everyday phenomena, from the visible to the microscopic. It powers the movement of everything from planets to playground swings. Consider the energy required for a bird to fly or a ball to roll down a hill; these are practical manifestations of kinetic energy at work.
Activation Energy Uncovered
Definition and Significance
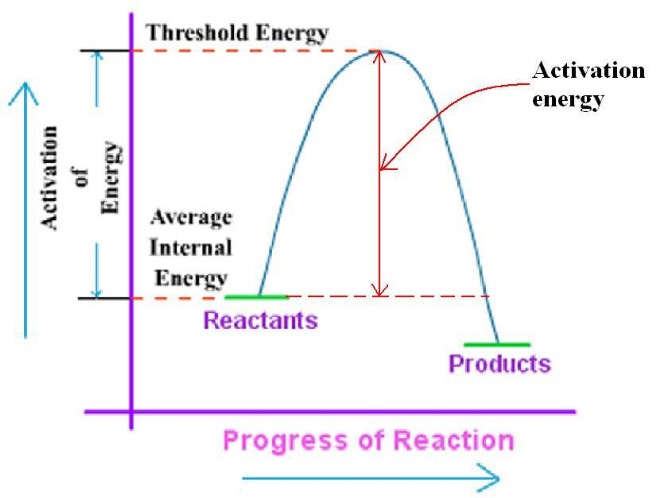
Activation energy is the minimum amount of energy required to start a chemical reaction. It’s a barrier that reactants must overcome for a reaction to occur. Without sufficient activation energy, chemical reactions cannot proceed, making it a critical factor in the study of chemistry and biology.
How It Relates to Chemical Reactions
Activation energy is essential for chemical reactions because it determines the speed and feasibility of reactions. Reactions with low activation energies occur more readily, while those with high activation energies may require the input of additional energy, like heat, to proceed.
Examples in Daily Life
Activation energy is at play in various everyday situations:
- Striking a Match: The friction provides the activation energy needed for the chemicals on the match head to ignite.
- Digesting Food: Enzymes lower the activation energy required for the biochemical reactions that break down food molecules.
Core Differences
Energy Types Comparison
While kinetic energy and activation energy are both critical concepts in physics and chemistry, they serve different purposes. Kinetic energy is about the energy of motion, relevant to objects and systems in motion. Activation energy, however, is specific to the field of chemistry, relating to the initiation of chemical reactions.
Impact on Physical and Chemical Processes
The impact of kinetic and activation energy extends across physical movements and chemical reactions. Kinetic energy influences the speed and force of moving objects, while activation energy determines whether chemical reactions occur and at what rate.
Visualization through Graphs
Graphical representations can help illustrate the differences and impacts of kinetic and activation energy. For instance, a graph showing a chemical reaction’s progress would highlight the energy peak representing the activation energy barrier that reactants must overcome.
Measurement Techniques
Methods for Kinetic Energy
Measuring kinetic energy often involves calculating the mass and velocity of an object. For more complex scenarios, such as rotational kinetic energy, instruments like gyroscopes and accelerometers may be used.
Approaches for Activation Energy
Activation energy measurements can be more complex, involving calorimetry or spectroscopy to analyze reaction rates and energy changes. Scientists may use:
- Calorimetry to measure the heat of reaction
- Spectroscopy for observing changes at the molecular level
Challenges and Solutions
Measuring these energies comes with challenges, from accurately determining an object’s velocity to isolating a reaction to measure its activation energy. Solutions include advanced technology and methodologies, improving accuracy and reliability in these measurements.
Factors Influencing Both
Temperature’s Role
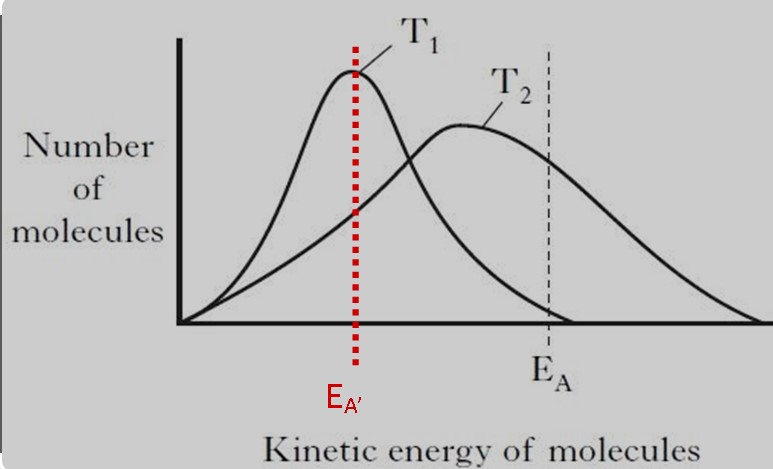
Temperature plays a critical role in both kinetic and activation energy. It directly influences the speed at which molecules move, thereby affecting their kinetic energy. Higher temperatures increase the velocity of molecules, leading to a rise in kinetic energy. Similarly, temperature can affect activation energy because it influences the energy distribution among reactants. In chemical reactions, higher temperatures often lower the effective activation energy required, facilitating the reaction by providing more molecules with sufficient energy to overcome the energy barrier.
Mass and Velocity for Kinetic Energy
The relationship between mass, velocity, and kinetic energy is foundational in physics. Here’s how they interact:
- Mass: An object’s mass is directly proportional to its kinetic energy. Doubling the mass of an object, assuming velocity remains constant, doubles its kinetic energy.
- Velocity: Velocity has a more significant impact on kinetic energy due to its quadratic relationship in the kinetic energy formula (��=12��2KE=21mv2). This means that doubling the velocity of an object increases its kinetic energy by a factor of four.
Catalysts and Activation Energy
Catalysts are substances that reduce the activation energy needed for a chemical reaction without being consumed in the process. They work by providing an alternative pathway for the reaction that has a lower energy barrier. This allows more reactants to have enough energy to overcome the activation energy barrier, thus increasing the reaction rate. Catalysts are fundamental in numerous industrial and biological processes due to their ability to efficiently drive reactions.
Practical Applications
Kinetic Energy in Engineering
In engineering, kinetic energy has diverse applications, from the design of vehicles and machinery to the generation of renewable energy. Engineers exploit the principles of kinetic energy in creating efficient systems where motion and energy transfer are key. For example, in the automotive industry, understanding kinetic energy is crucial for designing safer cars that can absorb energy effectively in a crash. Wind turbines convert the kinetic energy of wind into electrical energy, showcasing another practical application of kinetic energy in sustainable technology.
Activation Energy in Biochemistry
Activation energy is a cornerstone concept in biochemistry, particularly in the study of enzymes. Enzymes are biological catalysts that reduce the activation energy of biochemical reactions, which is essential for life processes. For instance, enzymes in the human body lower the activation energy required for metabolic reactions, allowing these reactions to proceed at a rate necessary for life. Understanding activation energy has led to significant advancements in medical science, such as the development of drugs that can inhibit or enhance enzyme activity.
Environmental Implications
Both kinetic and activation energy have important environmental implications. The kinetic energy of water in hydroelectric power plants is converted into electricity, providing a clean energy source. Conversely, the concept of activation energy is critical in understanding and controlling the rate of environmentally relevant chemical reactions, such as the breakdown of pollutants and greenhouse gases. Efficiently managing these energies can lead to more sustainable environmental practices.
Common Misunderstandings
Misconception about Energy Conversion
A common misconception is that energy can be lost or destroyed. However, according to the law of conservation of energy, energy cannot be created or destroyed; it can only be converted from one form to another. For example, when a car brakes, the kinetic energy of the car is converted into thermal energy, not lost. Understanding this principle is crucial for accurately discussing kinetic and activation energy.
Clarifying the Role in Reaction Speeds
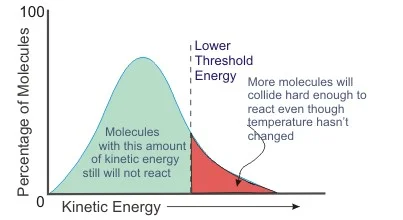
Another misunderstanding is regarding the role of activation energy in reaction speeds. It’s often thought that only increasing the activation energy can speed up reactions. In reality, reducing the activation energy, such as through the use of a catalyst, or increasing the temperature to provide more molecules with enough energy to overcome the activation energy barrier, are more effective ways to increase reaction rates.
Future Perspectives
Research Trends in Energy Study
The study of kinetic and activation energy is at the forefront of energy research. Scientists are exploring new materials and technologies to efficiently capture and utilize kinetic energy, such as piezoelectric materials that convert mechanical stress into electrical energy. In chemistry, research is focusing on developing novel catalysts that can lower activation energy for critical reactions, including those involved in carbon capture and storage to combat climate change.
Potential Technological Advancements
The future holds exciting potential technological advancements driven by our understanding of kinetic and activation energy:
- Smart materials: Materials that can efficiently convert kinetic energy from our environment into usable energy, powering devices and potentially reducing our reliance on traditional energy sources.
- Enzyme mimics: Synthetic catalysts designed to mimic enzyme activity, offering new ways to catalyze reactions with lower activation energies, leading to more efficient industrial processes and environmental remediation techniques.
Frequently Asked Questions
How is kinetic energy calculated?
Kinetic energy is calculated using the formula ��=12��2KE=21mv2, where �m is the mass of the object in kilograms and �v is the velocity of the object in meters per second. This formula highlights the direct relationship between an object’s mass, its speed, and the kinetic energy it possesses.
Why is activation energy important?
Activation energy is crucial because it determines the rate at which a chemical reaction will occur. A higher activation energy means a slower reaction rate, as fewer molecules have the necessary energy to overcome the energy barrier. This concept is vital in designing catalysts and controlling industrial chemical processes.
Can activation energy be negative?
Activation energy cannot be negative. It represents the energy barrier that must be overcome for a reaction to proceed. A negative value would imply that the reaction proceeds without any input of energy, which contradicts the principle that energy is needed to initiate most chemical transformations.
What affects kinetic energy?
The kinetic energy of an object is affected by its mass and velocity. An increase in either mass or velocity will result in a higher kinetic energy. This relationship underscores the principle that more massive objects moving at higher speeds possess greater energy, impacting everything from physical impacts to energy generation.
Conclusion
Kinetic energy and activation energy are cornerstones of understanding motion and reaction in the physical realm. Their distinct roles and mechanisms offer insight into the fundamental principles that govern the behavior of matter. As we continue to explore the intricacies of energy in science, these concepts serve as essential tools for unlocking new discoveries and technologies.
The journey through the realms of kinetic and activation energy highlights the profound impact these forces have on our world. From the swift motion of a sprinter to the transformative power of a chemical reaction, these energies shape the universe in intricate and fascinating ways, beckoning us to explore further the mysteries of the natural world.