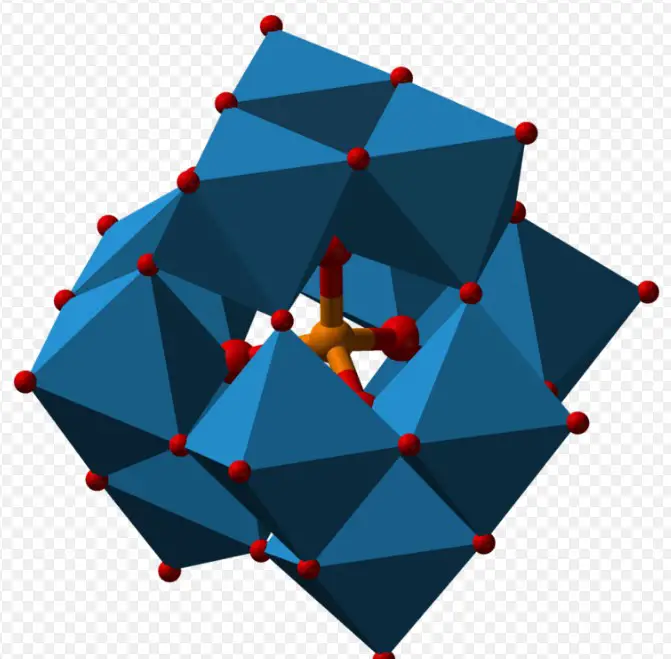
Polyoxometalates, the larger family to which both isopoly and heteropoly acids belong, represent a fascinating corner of inorganic chemistry that touches everything from industrial catalysis to medicine. These compounds are not only known for their diverse chemical structures but also for their remarkable physical and chemical properties, which make them invaluable in various scientific and technological applications.
Isopoly acids are formed when a metal atom is surrounded by oxygen atoms, creating a molecular structure that does not incorporate atoms of elements different from the metal and oxygen. In contrast, heteropoly acids involve a similar metal-oxygen framework but are distinguished by the inclusion of heteroatoms, such as phosphorus or silicon, into their structure. This fundamental difference in composition leads to distinct physical and chemical properties between the two types of acids.
The importance of these compounds extends beyond their intriguing molecular structures. In industrial applications, they serve as catalysts facilitating a variety of chemical reactions, while in the field of medicine, they show potential in drug delivery systems and as antiviral agents. The dual nature of isopoly and heteropoly acids, differing significantly in their chemical makeup and applications, highlights the versatility and complexity of inorganic chemistry in addressing practical challenges and advancing technology.
What Are Polyoxometalates?
Definition
Polyoxometalates (POMs) are a class of inorganic compounds characterized by their metal-oxygen clusters. These clusters consist of transition metals, most commonly molybdenum, tungsten, and vanadium, surrounded by oxygen atoms. The unique feature of POMs is their ability to form a wide variety of structures with different shapes, sizes, and charges.
Role in Catalysis, Medicine, and Materials Science
POMs play a pivotal role in several fields due to their versatile chemical properties.
- Catalysis: In catalysis, POMs are known for their acidic properties and redox potential. They can catalyze a range of reactions, including oxidation, hydrolysis, and organic transformations.
- Medicine: In the medical field, POMs have shown promise in antiviral, antibacterial, and anticancer therapies. Their ability to interact with biological molecules opens up new avenues for drug development.
- Materials Science: POMs contribute to materials science by offering electrochemical properties useful in energy storage devices, like batteries and supercapacitors. Their structural diversity also enables the development of novel materials with tailored properties.
Basics of Isopoly Acids
Definition and Formation
Isopoly acids are a type of POMs formed by the condensation of monomeric oxoanions of a single type of transition metal, such as molybdenum or tungsten. This process results in a polymeric structure that only includes metal and oxygen atoms, without any heteroatom.
Common Examples
- Molybdic Acid (H₂MoO₄): Formed from molybdenum.
- Tungstic Acid (H₂WO₄): Formed from tungsten.
These examples illustrate the simplicity of isopoly acids, focusing on a single metal type in their composition.
Properties
Isopoly acids exhibit several important properties:
- Solubility: Many isopoly acids are water-soluble, making them easy to use in aqueous solutions.
- Acidity: They act as acids in chemical reactions, donating protons to bases.
- Oxidation States: The transition metals in isopoly acids can adopt multiple oxidation states, influencing the compound’s reactivity and applications.
Composition and Structure
Atomic Structure
The atomic structure of isopoly acids consists of central metal atoms surrounded by oxygen atoms. The precise arrangement of these atoms determines the acid’s properties and reactivity.
Bonding and Geometry
- Bonding: The metal and oxygen atoms in isopoly acids are linked by covalent bonds, with oxygen acting as a bridge between metal centers.
- Geometry: The geometric arrangement can vary, forming structures like chains, rings, or cages, depending on the specific metal and conditions of formation.
Applications
Industrial Uses
Isopoly acids find applications in various industrial processes:
- Water Treatment: Used in the removal of pollutants and heavy metals from water.
- Electroplating: Serve as additives in electroplating solutions to improve the quality of coatings.
Role in Catalysis and Research
- Catalysis: Isopoly acids are effective catalysts for organic transformations, offering a green alternative to traditional catalysts.
- Research: They are also used in research for the development of new materials and understanding of chemical processes.
Basics of Heteropoly Acids
Definition and Formation
Heteropoly acids (HPAs) are a unique class of polyoxometalates that include both metal and non-metal atoms in their molecular framework. These acids are synthesized through the condensation of oxoanions of transition metals, such as molybdenum and tungsten, with the addition of heteroatoms like phosphorus or silicon. This process yields complex structures that have remarkable chemical versatility and reactivity.
Distinguishing Features from Isopoly Acids
Unlike isopoly acids, HPAs incorporate heteroatoms, which significantly alter their chemical and physical properties. This key difference enhances their catalytic efficiency and expands their applications beyond those of isopoly acids. The presence of heteroatoms in HPAs not only increases their acidity but also their structural diversity.
Common Examples
- Phosphomolybdic Acid (H₃PMo₁₂O₄₀): A combination of molybdenum, oxygen, and phosphorus.
- Silicotungstic Acid (H₄SiW₁₂O₄₀): Formed from tungsten, oxygen, and silicon.
These examples highlight the complex nature of heteropoly acids and their capacity for chemical customization.
Composition and Structure
Molecular Structure Differences
HPAs are characterized by their heterogeneous atomic composition, which results in a wide range of molecular structures. The inclusion of heteroatoms leads to the formation of Keggin, Dawson, and Anderson structures, among others. Each structure type has a distinct arrangement of metal and oxygen atoms around the central heteroatom.
Metal and Non-metal Atoms
The metal atoms in HPAs, typically molybdenum, tungsten, and vanadium, provide a framework that is stabilized by the presence of non-metal heteroatoms such as phosphorus or silicon. This unique combination of metal and non-metal atoms contributes to the versatility and reactivity of heteropoly acids.
Applications
Catalysis
HPAs are renowned for their role in catalysis, particularly in acid-catalyzed reactions and oxidation processes. Their high acidity and ability to undergo reversible redox reactions make them ideal catalysts for organic synthesis, petrochemical refining, and environmental remediation.
Medicine and Technology
In medicine, HPAs have shown potential in drug delivery systems and as antiviral agents. In technology, their electrochemical properties are being explored for use in energy storage, sensors, and photocatalysis.
Key Differences
Chemical Composition
The primary distinction between isopoly and heteropoly acids lies in their chemical composition. HPAs contain heteroatoms, which isopoly acids do not. This difference significantly affects their chemical behavior and applications.
Structural Characteristics
Framework and Geometry
HPAs exhibit a variety of geometrical structures, including Keggin and Dawson, determined by the type and arrangement of their metal and heteroatoms. This structural diversity is absent in isopoly acids, which have simpler geometries.
Bonding Differences
The bonding in HPAs is more complex due to the interaction between metal, oxygen, and heteroatoms. This leads to higher stability and reactivity compared to isopoly acids, which have straightforward metal-oxygen bonding.
Functional Applications
Catalytic Properties
HPAs possess superior catalytic properties, including enhanced acidity and redox activity, making them more effective in catalytic applications than isopoly acids.
Industrial and Medical Uses
The industrial applications of HPAs are vast, ranging from catalysis to waste treatment. In medicine, their biocompatibility and reactive nature offer promising avenues for therapeutic applications.
Significance in Research and Industry
Advancements in Catalysis
HPAs have led to significant advancements in catalysis, enabling more efficient and environmentally friendly chemical processes. Their high acidity and selectivity are crucial for innovations in organic synthesis and industrial chemistry.
Environmental Applications
In environmental science, HPAs play a vital role in pollution control and sustainable technologies. Their ability to catalyze the breakdown of pollutants and facilitate green chemistry processes is of paramount importance.
Future Prospects
The ongoing research into HPAs is expanding their potential applications in energy, medicine, and nanotechnology. The unique properties of HPAs continue to offer new solutions to complex problems, promising exciting developments for the future.
Frequently Asked Questions
What Are Polyoxometalates?
Polyoxometalates are a group of inorganic compounds characterized by an oxygen-metal framework. They are highly valued in various fields for their unique catalytic, redox, and structural properties, playing critical roles in catalysis, materials science, and medicine.
How Do Isopoly Acids Differ From Heteropoly Acids?
The main difference between isopoly and heteropoly acids lies in their composition. Isopoly acids consist solely of a metal and oxygen, forming a simple molecular structure, while heteropoly acids also include heteroatoms like phosphorus or silicon, which introduces complexity and alters their properties and applications.
Why Are Heteropoly Acids Important in Catalysis?
Heteropoly acids are pivotal in catalysis due to their strong acidity and the ability to undergo reversible redox reactions. Their structural flexibility allows for the optimization of catalytic sites, making them highly effective in facilitating a wide range of chemical reactions.
Can Polyoxometalates Be Used in Medicine?
Yes, polyoxometalates, including certain types of heteropoly acids, have shown potential in medical applications. Their ability to act as antiviral, antibacterial agents, and their use in drug delivery systems are areas of active research, highlighting their potential in therapeutic and diagnostic applications.
Conclusion
Isopoly and heteropoly acids represent two distinct classes of compounds within the fascinating world of polyoxometalates, each with its unique structural characteristics and applications. The exploration of these acids not only enriches our understanding of inorganic chemistry but also opens up new avenues for technological and scientific advancement. Their diverse roles, from catalyzing chemical reactions to potential medical applications, underscore the importance of these compounds in driving innovation across multiple disciplines.
As research continues to unveil the full potential of isopoly and heteropoly acids, it becomes clear that these compounds are more than just subjects of academic curiosity. They are vital tools in our ongoing quest to develop new technologies, improve existing processes, and create solutions to some of the most pressing challenges facing society today.