Chemical processes play a pivotal role in shaping the industrial landscape, with isomerization and aromatization standing out as key reactions in the field of organic chemistry. Both processes involve complex transformations of hydrocarbon molecules, yet their applications and implications differ significantly. By examining these processes, we gain insights into how basic elements are transformed into valuable chemical products.
Isomerization and aromatization are transformations that reconfigure molecules to either change their structure without altering the molecular formula, or to create aromatic rings, respectively. Isomerization alters the arrangement of atoms within a molecule, creating isomers with the same molecular formula but different physical and chemical properties. Aromatization, on the other hand, specifically converts non-aromatic rings into aromatic rings, known for their enhanced stability and reactivity due to delocalized electrons.
While isomerization often enhances fuel quality by increasing the octane number in gasoline, aromatization is crucial in synthesizing chemicals that form the basis for pharmaceuticals, dyes, and plastics. These processes not only contribute significantly to modern conveniences but also pose challenges and opportunities in terms of efficiency and environmental impact.
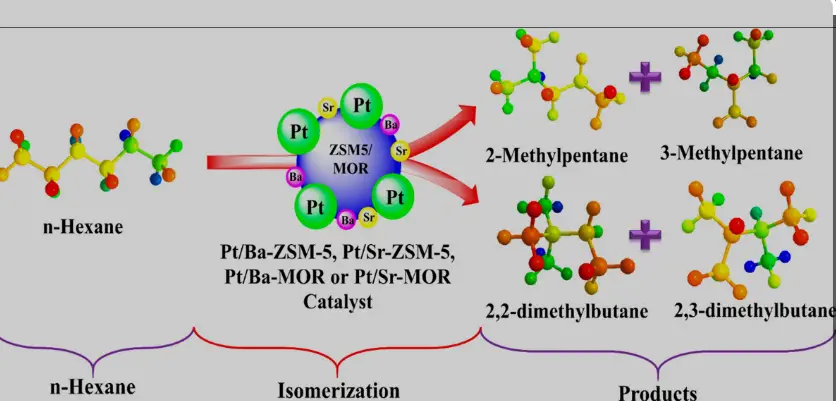
Basics of Isomerization
Definition of Isomerization
Isomerization is a chemical reaction where molecules with the same molecular formula are rearranged into different structural forms called isomers. This transformation changes the arrangement of atoms within a molecule, without altering its atomic composition. This reaction is critical for enhancing the properties of chemical compounds in various applications, notably in fuel production.
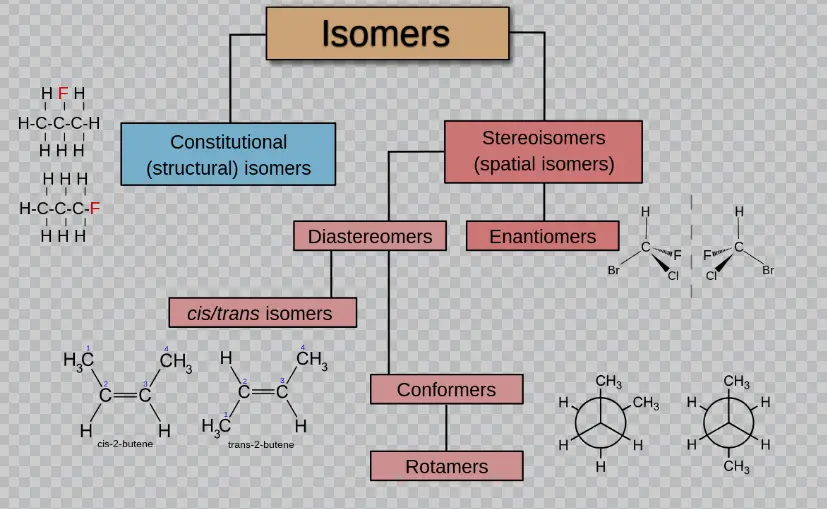
Types of Isomers
There are several types of isomers that result from isomerization, each varying in complexity and properties:
- Structural Isomers: These differ in the placement of their atoms and the connections between them.
- Stereoisomers: These isomers have the same bonds but differ in the spatial arrangements of their atoms. This category further divides into:
- Geometric Isomers: They differ in their arrangement around a double bond or a ring structure.
- Optical Isomers: These are mirror images of each other and differ in the way they rotate polarized light.
Common Isomerization Processes
Isomerization processes are prevalent in various industrial settings, especially in refining petroleum to enhance the quality of gasoline:
- Butane Isomerization: Converts n-butane into isobutane, a precursor for the production of alkylate, an additive in gasoline.
- Pentane and Hexane Isomerization: Improves the octane rating of gasoline by altering pentane and hexane into forms that burn more efficiently.
Basics of Aromatization
Definition of Aromatization
Aromatization is a chemical reaction in which non-aromatic hydrocarbons are transformed into aromatic compounds. Aromatic compounds are characterized by their stable ring-like structure, which includes delocalized electrons.
Role in Hydrocarbon Chemistry
Aromatization plays a pivotal role in hydrocarbon chemistry by creating compounds that are more stable and reactive. These compounds are essential in the synthesis of plastics, synthetic fibers, and pharmaceuticals, making aromatization a cornerstone of industrial chemistry.
Aromatization Reaction Examples
Several key reactions illustrate the application and importance of aromatization:
- Cyclization of Alkanes: Converts long-chain alkanes into cycloalkanes and subsequently into aromatic hydrocarbons.
- Dehydrogenation of Cyclohexanes: Turns cyclohexanes into benzene, a primary aromatic hydrocarbon used widely in chemical synthesis.
Chemical Structures Involved
Structural Changes in Isomerization
During isomerization, the main structural changes involve the rearrangement of carbon skeletons and shifting of functional groups within the molecule. These changes are crucial for altering the physical and chemical properties of the substance without changing its molecular formula.
Structural Changes in Aromatization
Aromatization typically involves the formation of a planar, cyclic structure of atoms with conjugated pi electron systems. This structural alteration leads to increased stability and reactivity, which are highly valued in various chemical synthesis processes.
Key Molecular Differences
The key differences between the molecular structures of isomers and aromatic compounds are primarily in stability and electron configuration. Aromatic molecules exhibit enhanced stability due to their resonance structures, while isomers might show different physical and chemical properties despite having the same molecular formula.
Catalysts and Conditions
Catalysts in Isomerization
Isomerization often requires catalysts to proceed efficiently. Common catalysts include:
- Acidic Catalysts: Such as alumina and silica, which help in rearranging the molecular structure.
- Platinum and Palladium: Metals that aid in the rearrangement of atoms within hydrocarbons.
Catalysts in Aromatization
Aromatization reactions typically use metal catalysts that facilitate the dehydrogenation necessary to form aromatic rings. Common catalysts include:
- Zeolites: Particularly ZSM-5, a type of zeolite used for converting alkanes to aromatic compounds.
- Molybdenum and Platinum: These metals are used to catalyze the dehydrogenation steps in aromatization.
Reaction Conditions Comparison
The reaction conditions for isomerization and aromatization vary significantly. Isomerization requires mild to moderate temperatures and pressures, often with a strong acidic medium. Aromatization, however, generally operates at higher temperatures and may also involve higher pressures, depending on the substrate and desired end products. Both processes need precise control over reaction parameters to optimize yield and selectivity towards the desired products.
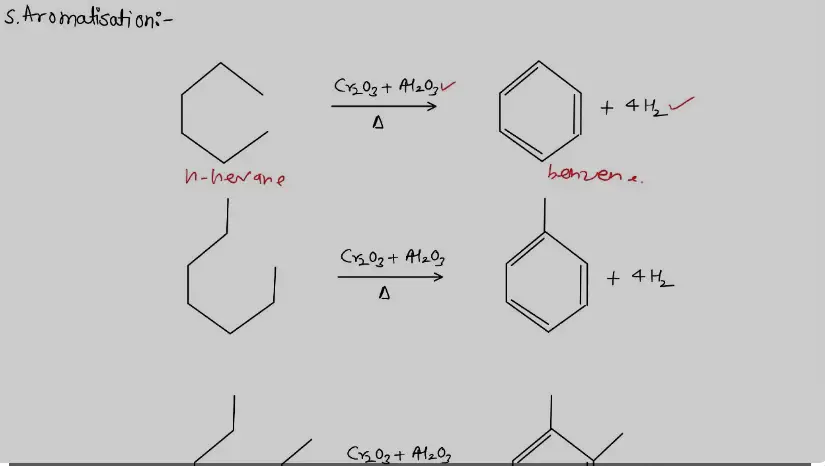
Industrial Applications
Isomerization in Industry
Isomerization has broad applications across various sectors, primarily in refining petroleum. It is instrumental in improving the quality of gasoline, making fuels burn more efficiently and cleanly. Isomerization units are standard in oil refineries, where light hydrocarbons such as butane and pentane are converted into isobutane and isopentane. These compounds significantly enhance the octane rating of gasoline, contributing to better engine performance and reduced emissions.
Aromatization in Industry
Aromatization finds its significance in the chemical industry, where it serves as a backbone for producing aromatic compounds used in a multitude of products. From the manufacture of plastics and synthetic fibers to pharmaceuticals and dyes, the applications are vast. Benzene, toluene, and xylene, known collectively as BTX, are produced through aromatization and are crucial for making a variety of consumer goods.
Impact on Product Quality
Both isomerization and aromatization have profound impacts on product quality:
- Isomerization enhances fuel stability and reduces engine knocking, a critical factor in automotive performance.
- Aromatization contributes to the production of chemicals with high purity and specific properties, necessary for high-quality plastics and other synthetic materials.
Environmental Impact
Emissions from Isomerization
Isomerization processes can release various emissions, including VOCs (Volatile Organic Compounds) and greenhouse gases. These emissions are a concern due to their impact on air quality and contribution to global warming.
Emissions from Aromatization
Similar to isomerization, aromatization can lead to the release of significant amounts of CO2 and other harmful substances. The process often involves the use of energy-intensive technologies that contribute to carbon footprints.
Mitigation Strategies
To combat these environmental effects, several strategies are employed:
- Advanced Catalysts: Developing more efficient catalysts that reduce unwanted byproducts and enhance reaction selectivity.
- Process Optimization: Implementing techniques that minimize energy consumption and maximize yield.
- Emission Controls: Utilizing technologies such as carbon capture and storage (CCS) to reduce the impact of harmful emissions.
Technological Advances
Recent Innovations in Isomerization
Recent technological advancements have focused on enhancing the efficiency and environmental friendliness of isomerization processes. New catalyst formulations and reactor designs have been developed to increase yield and minimize energy use.
Recent Innovations in Aromatization
In aromatization, innovations include the development of novel zeolite catalysts that are more selective and resistant to deactivation. These advancements help in producing higher-quality aromatic compounds while reducing side reactions that could lead to environmental pollution.
Future Prospects
The future of isomerization and aromatization looks promising with ongoing research into sustainable practices. The focus is increasingly shifting towards green chemistry approaches, aiming to reduce environmental impacts and improve economic outcomes.
Economic Implications
Economic Impact of Isomerization
Isomerization holds substantial economic benefits for the petroleum industry by allowing refineries to meet fuel standards and market demands more effectively. This process helps in maximizing the output of high-octane fuels from lighter components of crude oil, which are less valuable otherwise.
Economic Impact of Aromatization
Aromatization significantly influences the chemical industry’s economy, driving the production of aromatics that are precursors to numerous high-value products. The global demand for aromatics is driven by their applications in growing industries such as automotive, construction, and electronics.
Cost-Benefit Analysis
When evaluating the cost-effectiveness of these processes:
- Isomerization proves beneficial by turning lower-value hydrocarbons into high-value end products, thereby justifying the investment in technology and catalysts.
- Aromatization, while costly due to its high energy and catalyst requirements, offers returns that outweigh the initial investments, given the value of the products generated.
FAQs
What is Isomerization?
Isomerization is a chemical reaction where molecules with the same molecular formula undergo structural rearrangements. This process is crucial in petroleum refining, where it improves the octane rating of fuel, enhancing its quality and combustion efficiency.
How does Aromatization occur?
Aromatization involves the transformation of non-aromatic hydrocarbons into aromatic compounds. This is achieved through dehydrogenation, where hydrogen atoms are removed, leading to the formation of a stable aromatic ring, a structure with delocalized electrons.
Why is Aromatization important in industry?
Aromatization holds significant industrial importance as it produces aromatic hydrocarbons, which are key precursors for synthetic fibers, plastics, and various chemicals. The ability to create stable and reactive aromatic rings makes aromatization vital for the chemical manufacturing sector.
What are the environmental impacts of Isomerization?
Isomerization processes can contribute to environmental pollution through the release of greenhouse gases and other emissions. However, advancements in catalyst design and process optimization are continually reducing these impacts, making isomerization more environmentally friendly.
Can Aromatization be reversed?
Aromatization is typically a non-reversible chemical reaction. Once aromatic rings are formed, their stability due to delocalized pi-electrons makes them resistant to converting back to non-aromatic forms without significant energy input or specific conditions.
Conclusion
The exploration of isomerization and aromatization reveals their indispensable roles in modern chemistry and industry. These processes not only facilitate the production of high-quality fuels and essential chemicals but also challenge scientists and engineers to refine these reactions for greater efficiency and sustainability. As research progresses, further advancements are expected to enhance the utility and environmental compatibility of these vital chemical transformations.
In summary, understanding and improving isomerization and aromatization processes remain critical for advancing many sectors, including energy and pharmaceuticals. The ongoing evolution of these techniques promises to bring even more effective solutions to both existing and emerging challenges in chemical manufacturing and beyond.