Isocyanates and diisocyanates are crucial chemicals widely used in various industrial applications, including the production of foams, fibers, and coatings. These compounds are known for their reactivity and functionality, playing a pivotal role in the creation of polyurethane products. However, despite their importance, the distinct differences between isocyanate and diisocyanate are not widely understood outside of chemical manufacturing circles.
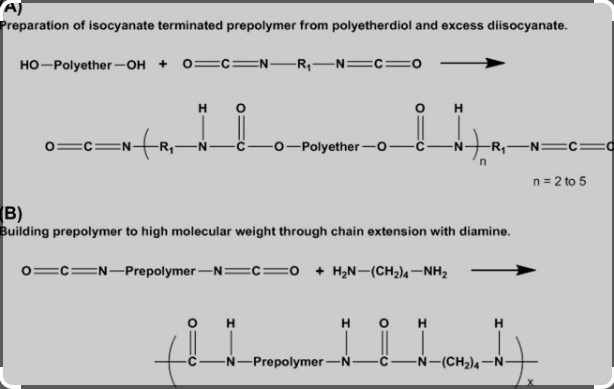
Isocyanates consist of a single isocyanate group attached to a radical, whereas diisocyanates contain two isocyanate groups. This structural variation significantly impacts their reactivity and the types of polymers they can produce. Diisocyanates, for instance, are more commonly used in the creation of rigid and flexible foams due to their cross-linking capabilities.
In the industrial landscape, these chemicals are not just raw materials; they are foundational to the manufacture of durable, versatile, and innovative products that meet rigorous performance and safety standards. Their applications range from simple household items to complex automotive components, highlighting their integral role in modern manufacturing processes.
Chemical Basics
Definition of Isocyanate
Isocyanates are chemical compounds containing the isocyanate group (-NCO). These compounds react with alcohols to form polyurethanes, which are used extensively in various industrial products. The functional group in isocyanates is highly reactive, allowing for easy bonding with other chemicals, particularly those containing hydroxyl groups.
Definition of Diisocyanate
Diisocyanates are a subset of isocyanates featuring two isocyanate groups in their molecular structure. This characteristic makes them doubly reactive compared to their monoisocyanate counterparts, significantly enhancing their utility in creating more complex polyurethane structures. Diisocyanates are critical in manufacturing foams, elastomers, and coatings.
Structural Differences
Molecular Structure of Isocyanate
The basic structure of an isocyanate involves a single isocyanate group attached to an organic radical. This simple structure is versatile yet limits the compound to certain types of reactions and products.
Molecular Structure of Diisocyanate
In contrast, diisocyanates contain two isocyanate groups. This arrangement not only increases their reactivity but also allows them to cross-link, creating three-dimensional polymer networks that are tougher and more durable.
Comparison of Structures
Comparing these structures, the key difference lies in the number of reactive sites: diisocyanates offer two, doubling their ability to form bonds with other compounds. This structural difference fundamentally changes how they behave in chemical reactions and affects the types of materials they can produce.
Production Processes
How Isocyanates are Produced
Isocyanates are typically produced by the reaction of amines with phosgene, a process that involves careful handling due to the toxic nature of phosgene. Steps include:
- Preparation: Synthesizing the amine precursor
- Reaction: Introducing phosgene to form the isocyanate
- Purification: Refining the product to achieve high purity
How Diisocyanates are Produced
Diisocyanates follow a similar production path but require a more complex setup to accommodate the double bonding potential. Their production also involves:
- Double synthesis stages: Ensuring that both isocyanate groups form correctly without unwanted reactions
- Controlled reaction environments: Maintaining precise conditions to prevent premature polymerization
Key Differences in Production
The main difference in production lies in the complexity and control required for diisocyanates. Their production is more sensitive to conditions and timing due to the dual reactive sites.
Common Uses
Uses of Isocyanates in Industry
Isocyanates are widely used in the manufacture of:
- Foams: Soft to rigid foams for furniture and insulation
- Adhesives and Sealants: High-performance products for automotive and construction industries
- Coatings: Protective and decorative coatings for a variety of surfaces
Uses of Diisocyanates in Industry
Diisocyanates extend to similar applications but are particularly dominant in:
- Rigid Foams: Essential for insulation in buildings and refrigeration systems
- Elastomers: Used in automotive parts, such as gaskets and seals, where flexibility and durability are crucial
Comparative Analysis of Applications
While both chemicals are used in similar sectors, diisocyanates are typically chosen for applications requiring stronger, more durable bonds and structures. Their ability to form cross-linked polymers makes them ideal for products that must withstand more stress or require greater longevity.
Health and Safety Concerns
Health Risks of Isocyanates
Isocyanates pose significant health risks primarily due to their high reactivity, which can cause adverse effects when inhaled or when they come into contact with skin. Key health issues include:
- Respiratory problems: Asthma-like symptoms can develop, which might become chronic.
- Skin irritation: Direct contact can lead to dermatitis and other skin conditions.
- Sensitization: Repeated exposure may lead to sensitization, increasing the risk of severe allergic reactions.
Health Risks of Diisocyanates
Diisocyanates share similar health risks with monoisocyanates but with increased severity due to their higher reactivity. This can lead to:
- More intense respiratory issues, potentially leading to occupational asthma.
- Increased risk of severe dermatitis and chemical burns upon contact.
Safety Measures for Handling
To mitigate these risks, several safety measures are critical:
- Proper ventilation: Ensuring good air quality in workplaces.
- Personal protective equipment (PPE): Gloves, masks, and eye protection.
- Training and protocols: Regular training on handling procedures and emergency responses.
Environmental Impact
Environmental Risks Associated with Isocyanates
Isocyanates, if not handled properly, can contribute to environmental pollution. Their volatility and toxicity can affect:
- Air quality: Release of volatile organic compounds (VOCs).
- Water pollution: Contamination of water sources through improper disposal.
Environmental Risks Associated with Diisocyanates
Similar to isocyanates, diisocyanates can also impact the environment, with potential effects being somewhat more pronounced due to their dual reactive nature. Key concerns include:
- Greater potential for long-lasting contamination in both soil and water bodies.
- Challenges in degradation, leading to persistence in the environment.
Sustainability Considerations
To address these issues, industries are focusing on:
- Recycling and recovery: Developing methods to reclaim and reuse these chemicals.
- Alternative materials: Researching less harmful substitutes.
- Regulatory compliance: Adhering to environmental laws to minimize impacts.
Market Trends
Demand Trends for Isocyanates
The market for isocyanates has seen steady growth, driven by demand in sectors such as:
- Automotive: For foams and sealants.
- Construction: For insulation and adhesives.
- Furniture: For flexible foam production.
Demand Trends for Diisocyanates
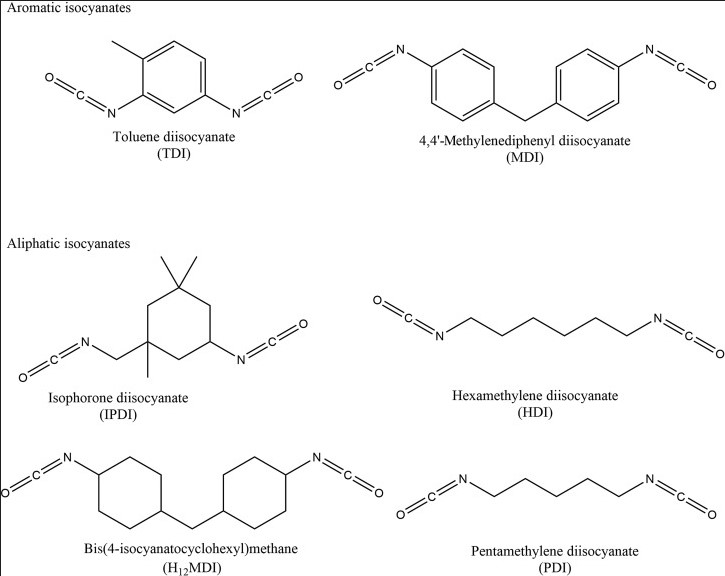
Diisocyanates, particularly MDI (methylene diphenyl diisocyanate) and TDI (toluene diisocyanate), have witnessed a surge in demand due to their extensive use in:
- Rigid foams: Crucial for energy-efficient building solutions.
- Elastomers: In various consumer products and automotive components.
Future Market Predictions
The future looks promising for both isocyanates and diisocyanates with anticipated expansions in:
- Emerging markets: Increased infrastructure projects in developing countries.
- Technological advancements: Innovations leading to new applications and products.
Legal and Regulatory Framework
Regulations Governing Isocyanates
Strict regulations are in place to control the use and disposal of isocyanates, aiming to protect both human health and the environment. These include:
- OSHA standards: For workplace exposure limits.
- EPA guidelines: For emissions and waste management.
Regulations Governing Diisocyanates
Similar to isocyanates, diisocyanates are subject to stringent regulations. Additional measures often apply due to their increased reactivity and potential for harm.
Impact of Regulations on Usage
Regulatory frameworks have led to:
- Safer workplace practices: Enhanced safety protocols and monitoring.
- Innovation in production: Development of less toxic and more environmentally friendly alternatives.
Frequently Asked Questions
What are Isocyanates?
Isocyanates are a family of highly reactive chemicals commonly used in the manufacture of polyurethanes. They contain at least one isocyanate group (-NCO) and are known for their ability to react with compounds containing alcohol (hydroxyl) groups to form polyurethanes.
How are Diisocyanates different from Isocyanates?
Diisocyanates are a type of isocyanate that contain two isocyanate groups instead of one. This allows them to form more complex polymers, making them essential in producing a variety of polyurethane products, including rigid and flexible foams, coatings, adhesives, and sealants.
What are the risks of handling Isocyanates?
Handling isocyanates poses significant health risks such as respiratory problems, skin irritation, and sensitization. Adequate safety measures, including the use of personal protective equipment and proper ventilation, are crucial to minimize these risks.
Where are Diisocyanates used?
Diisocyanates are primarily used in the production of polyurethane products like foams, which are prevalent in automotive, furniture, and insulation applications. Their ability to create strong, durable bonds makes them invaluable in these industries.
Conclusion
Isocyanates and diisocyanates are foundational to the polyurethane industry, driving innovation and functionality in numerous products we use daily. Understanding their distinct properties and applications not only enhances industrial efficiency but also promotes safer handling practices.
The chemical nuances between isocyanate and diisocyanate highlight the importance of specialized knowledge in their application and safety management. As industries continue to evolve, so too will the methods to utilize these vital chemicals safely and effectively, ensuring they continue to play a crucial role in our technological and material advancements.

