Solids are a fundamental part of our everyday lives, shaping the world around us in countless ways. From the salt in our food to the metals that form the backbone of our cities, understanding the differences between ionic and metallic solids is crucial for appreciating how materials behave and are utilized in various applications. These two types of solids differ significantly in their properties and applications, influenced by their unique bonding and structural characteristics.
Ionic and metallic solids are distinguished primarily by their bonding: ionic solids are formed through the electrostatic attraction between ions, while metallic solids are characterized by a sea of delocalized electrons surrounding positive ions. This distinction leads to a variety of different properties, including differences in electrical conductivity, melting and boiling points, hardness, and solubility.
Ionic solids, such as table salt, are often brittle and have high melting points, while metallic solids, like iron, are malleable and conduct electricity well. These differences stem from their atomic and molecular structures, influencing not only their physical and chemical properties but also their practical applications in industries ranging from electronics to construction.
Basics of Ionic Solids
Definition and Characteristics
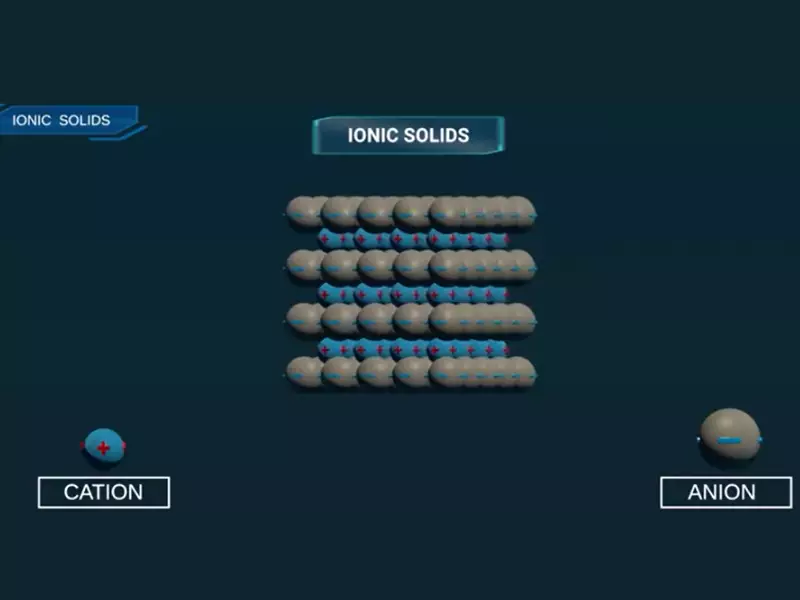
Ionic solids are a class of compounds characterized by their electrostatic forces between ions. These forces arise from the attraction between positively charged ions, known as cations, and negatively charged ions, or anions. The result is a solid that tends to be hard, brittle, and with high melting points. Ionic solids are also known for their ability to conduct electricity when molten or dissolved in water, due to the movement of ions.
Formation and Bonding
The formation of ionic solids occurs through the transfer of electrons from metal atoms to non-metal atoms. This transfer creates ions: metals lose electrons to become cations, while non-metals gain electrons to become anions. The electrostatic attraction between these oppositely charged ions forms an ionic bond, creating a crystalline lattice that holds the solid together.
Examples
Common examples of ionic solids include table salt (NaCl), potassium bromide (KBr), and calcium chloride (CaCl2). Each of these compounds features a metal ion and a non-metal ion, joined by ionic bonds to form a solid with distinctive ionic properties.
Basics of Metallic Solids
Definition and Characteristics
Metallic solids are materials made of metal atoms held together by metallic bonds. These bonds are characterized by a sea of delocalized electrons that flow freely around the metal ions. This unique bonding arrangement gives metallic solids their notable properties, such as high electrical and thermal conductivity, malleability, ductility, and luster.
Formation and Bonding
Metallic bonding occurs when metal atoms share their outermost electrons freely with many other atoms. This sharing creates a sea of electrons that surrounds positive metal ions in a lattice. The delocalized nature of these electrons allows them to move freely, contributing to the solid’s ability to conduct electricity and heat.
Examples
Examples of metallic solids include iron (Fe), gold (Au), copper (Cu), and aluminum (Al). These metals, along with many others, demonstrate the typical physical properties associated with metallic bonding, including their shiny appearance and ability to deform without breaking.
Key Differences
Bonding Nature
Ionic Solids
Ionic solids are characterized by electrostatic forces between ions. This bonding occurs due to the attraction between cations and anions, leading to a structure that is rigid and brittle.
Metallic Solids
In contrast, metallic solids feature a sea of electrons. This type of bonding results from the sharing of electrons among a lattice of metal ions, giving rise to properties like conductivity and malleability.
Electrical Conductivity
Ionic Solids
In their solid state, ionic compounds do not conduct electricity. However, when melted or dissolved in water, they become ionic conductors due to the mobility of their ions.
Metallic Solids
Metallic solids, on the other hand, are excellent conductors of electricity in their solid state. The free electrons in the metal lattice allow for easy flow of charge, making metals ideal for electrical applications.
Melting and Boiling Points
Ionic solids typically exhibit higher melting and boiling points compared to metallic solids. The strong ionic bonds in these compounds require more energy to break, leading to higher temperatures needed for melting and boiling.
Metallic solids, with their more flexible metallic bonds, tend to have lower melting and boiling points. The ease with which metal atoms can slide past each other underlines their lower temperature thresholds.
Hardness and Brittleness
Ionic Solids
Ionic solids are hard but brittle. The rigid ionic lattice can crack or shatter under stress due to the alignment of ions.
Metallic Solids
Conversely, metallic solids exhibit malleability and ductility. These properties allow metals to be hammered into thin sheets or drawn into wires, a testament to the flexibility of metallic bonds.
Solubility
Ionic Solids
Ionic compounds are generally soluble in water. Water molecules can surround and separate the ions, leading to the dissolution of the solid.
Metallic Solids
Metallic solids do not dissolve in water. Their bonding structure does not allow for easy separation by water molecules, making them insoluble.
Magnetic Properties
Metallic Solids
Some metallic solids possess magnetic properties due to the alignment of their electron spins. This feature is absent in ionic solids, highlighting another distinction between the two types of materials.
Applications
Ionic Solids
Use in Everyday Life and Industry
Ionic solids play a crucial role in both our daily lives and various industrial applications. Their unique properties, such as high melting points, solubility in water, and electrical conductivity when molten, make them indispensable in numerous fields. From the food we eat to the products we use for cleaning and maintenance, ionic compounds are everywhere.
In everyday life, table salt (NaCl) is perhaps the most familiar ionic solid. It is not just a dietary necessity for humans; it also serves as a fundamental preservative, dating back to ancient times when refrigeration was non-existent. Beyond culinary uses, ionic compounds like sodium bicarbonate (baking soda) play a part in baking, cleaning, and even personal hygiene products.
In industry, ionic solids find applications in a vast array of processes and products. Calcium chloride (CaCl2), for example, is used for ice and dust control on roads. It is also a key ingredient in the manufacture of plastics and in the paper industry to increase web strength. Another ionic compound, sodium hydroxide (NaOH), is crucial in the production of soap, paper, and various dyes.
Specific Examples
- Water Softening: Ionic solids like sodium carbonate (Na2CO3) are used to soften water by precipitating calcium and magnesium ions, which cause hardness.
- Agriculture: Fertilizers are rich in ionic compounds such as potassium nitrate (KNO3), providing essential nutrients to plants.
- Medicine: Many medications are in the form of ionic compounds, including lithium carbonate (Li2CO3) for bipolar disorder and sodium fluoride (NaF) in toothpaste to prevent dental cavities.
Metallic Solids
Use in Technology and Construction
Metallic solids are the backbone of modern technology and construction. Their excellent electrical conductivity, strength, malleability, and ductility make them the material of choice for a wide range of applications. From building structures to the intricate circuits in electronic devices, metals are fundamental to our modern world.
In technology, metals like copper (Cu) and gold (Au) are indispensable due to their outstanding electrical conductivity. Copper is widely used in electrical wiring, electronics, and motors, while gold’s resistance to corrosion and excellent conductivity make it ideal for precision electronics, such as connectors in smartphones and computers.
The construction industry relies heavily on metallic solids such as steel—an alloy of iron (Fe) and carbon (C)—for its strength and versatility. Steel is used in everything from the framework of skyscrapers and bridges to the fabrication of tools and vehicles. Aluminum (Al), known for its light weight and resistance to corrosion, is used in the construction of buildings, airplanes, and lightweight vehicles.
Specific Examples
- Electronics: Metals like silver (Ag), with the highest electrical conductivity of any element, are used in high-end audio and video connections for unparalleled clarity and performance.
- Renewable Energy: Metallic solids such as lithium (Li) play a key role in the batteries that power electric vehicles and store energy from renewable sources.
- Infrastructure: Titanium (Ti) alloys, known for their high strength-to-weight ratio and corrosion resistance, are crucial in the construction of modern aircraft and spacecraft.
Frequently Asked Questions
What causes the differences in electrical conductivity between ionic and metallic solids?
Ionic solids conduct electricity in molten form or when dissolved in water, as their ions are free to move. In contrast, metallic solids conduct electricity in the solid state due to the mobility of their delocalized electrons, allowing them to act as charge carriers.
Why are ionic solids brittle and metallic solids malleable?
The brittleness of ionic solids arises from the strong electrostatic forces between oppositely charged ions, which, when displaced, can cause the solid to crack or shatter. Metallic solids are malleable because their delocalized electrons allow metal atoms to slide past one another without breaking the metallic bond.
Can metallic solids dissolve in water like ionic solids?
Generally, metallic solids do not dissolve in water. This is because the metallic bonding that holds the atoms together in a metal is not easily broken by the interactions with water molecules, unlike the ionic bonds in ionic solids that can be disrupted by water to dissolve.
How do melting points of ionic and metallic solids compare?
Ionic solids typically have higher melting points than metallic solids due to the strong ionic bonds between their positive and negative ions. Metallic bonds, while strong, allow for a more flexible structure that generally requires less energy to break apart, resulting in lower melting points for metallic solids.
Conclusion
The distinctions between ionic and metallic solids reveal much about how materials interact with the world and serve various purposes in technology, construction, and daily life. Understanding these differences is not just academic but practical, as it guides the selection of materials for specific applications based on their electrical conductivity, hardness, melting points, and other critical properties.
In summary, the comparison between ionic and metallic solids illustrates the incredible variety of materials available to us, each with its unique set of properties and applications. By exploring the nuances of these solids, we unlock a deeper understanding of the materials that make up the world around us, enhancing both our appreciation for and our ability to innovate within the realm of material science.
