Molecular interactions are the unseen forces that choreograph the dance of atoms and molecules in the vast stage of chemistry and biology. These interactions, subtle yet powerful, determine the structure, properties, and behaviors of matter, from the simplest compounds to the complex machinery of biological life. Among these, ionic and electrostatic interactions play critical roles, guiding the formation of compounds, influencing molecular stability, and driving biological processes.
Ionic interactions occur when atoms transfer electrons to achieve a more stable electron configuration, forming ions that are positively or negatively charged. These ions attract each other to form ionic bonds, a fundamental force in the creation of salts and minerals. Electrostatic interactions, on the other hand, involve the attraction and repulsion between charged particles, including ions, molecules, and atoms, influencing everything from the solubility of substances to the shape and function of biological macromolecules.
While both types of interactions are central to understanding chemical and biological systems, they differ in their mechanisms, effects, and applications. Ionic interactions result from the transfer of electrons and the formation of ionic bonds, leading to the creation of compounds with distinct properties like high melting points and electrical conductivity. Electrostatic interactions, encompassing a range of forces including dipole-dipole interactions and hydrogen bonds, play a pivotal role in the physical properties of substances, molecular recognition processes, and the stability of complex structures like proteins and DNA.
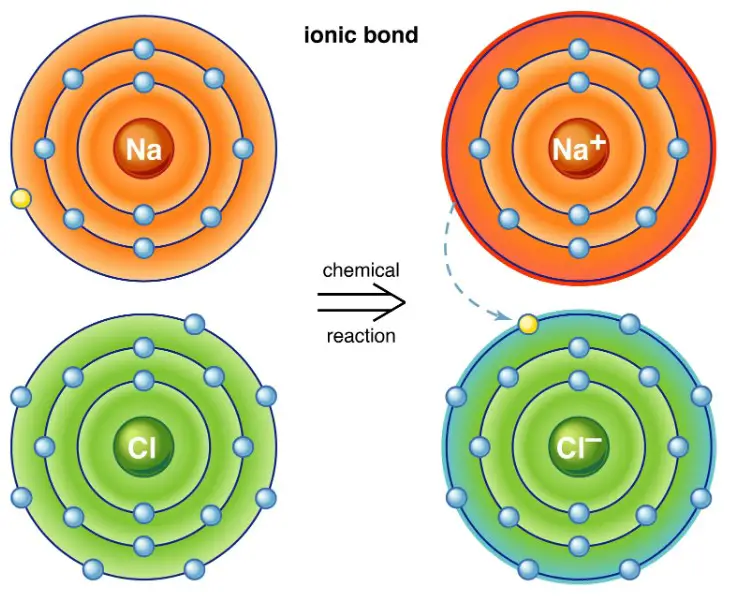
Ionic Interactions
Definition and Basic Concepts
Ionic interactions are fundamental forces in chemistry and biology, shaping the properties and behaviors of substances at the molecular level. They occur when atoms transfer electrons to or from other atoms, creating ions. These ions, which carry positive or negative charges, attract each other, forming ionic bonds. This attraction is a cornerstone of the structure and stability of many compounds.
Examples in Real-Life and Scientific Applications
Ionic interactions play a crucial role in both everyday substances and advanced scientific applications. For example:
- Table salt (NaCl): Perhaps the most familiar ionic compound, it forms when sodium (Na) donates an electron to chlorine (Cl), creating positively charged sodium ions and negatively charged chloride ions.
- Batteries: Lithium-ion batteries rely on the movement of ions between the anode and cathode to store and release energy.
- Water Softeners: These devices use ionic interactions to remove calcium and magnesium ions from hard water, replacing them with sodium ions.
Role in Biological Systems
In biology, ionic interactions are essential for the structure and function of biomolecules. They contribute to the stability of proteins and DNA, enable nerve impulse transmission, and are involved in the transport of substances across cell membranes.
Formation
How Ionic Bonds Form
Ionic bonds form through a step-by-step process:
- Electron Transfer: An atom loses one or more electrons, becoming a positively charged ion, while another atom gains those electrons, becoming a negatively charged ion.
- Attraction: The oppositely charged ions attract each other.
- Bond Formation: The attraction holds the ions together in a stable arrangement, forming an ionic compound.
Factors Influencing Ionic Bond Strength
Several factors affect the strength of ionic bonds, including:
- Charge Magnitude: Ions with higher charges attract each other more strongly.
- Ion Size: Smaller ions can get closer together, increasing the attraction force.
- Environmental Conditions: The presence of water or other substances can weaken ionic bonds by interfering with the ionic attraction.
Characteristics
Strength and Stability
Ionic bonds are known for their strength and stability, resulting from the strong electrostatic forces between oppositely charged ions. This makes ionic compounds have high melting and boiling points, and makes them generally soluble in water but not in non-polar solvents.
Ionic Compounds: Properties and Examples
Ionic compounds exhibit a range of distinct properties:
- High Melting and Boiling Points: Due to the strong attractions between ions.
- Electrical Conductivity: In molten or dissolved state, ionic compounds can conduct electricity.
- Brittleness: Ionic crystals are hard but brittle because the strong forces holding them together can cause them to shatter if struck.
Examples of ionic compounds include sodium chloride (NaCl), potassium iodide (KI), and calcium carbonate (CaCO3).
Biological Significance
Ionic Interactions in Proteins and DNA
In biological systems, ionic interactions are vital for the structure and function of proteins and DNA:
- Proteins: Ionic bonds between amino acid side chains help stabilize the protein’s three-dimensional structure, affecting its activity and interaction with other molecules.
- DNA: Ionic interactions between the phosphate groups of the DNA backbone and surrounding water or proteins help compact and stabilize the DNA structure.
Impact on Structure and Function of Biomolecules
The presence and strength of ionic interactions in biomolecules significantly impact their structure, stability, and function. For example, the folding of proteins into their functional forms is partly driven by ionic interactions, which also play a crucial role in the binding of substrates to enzymes and the recognition of signaling molecules by receptors. In DNA, ionic interactions are critical for maintaining the double helix structure and for the binding of regulatory proteins.
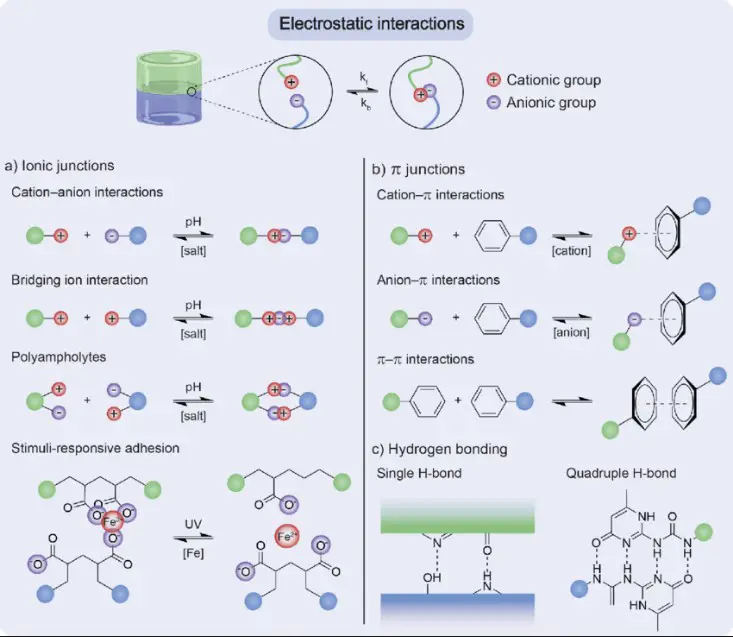
Electrostatic Interactions
Definition and Principles
Electrostatic interactions refer to the forces between charged particles that are either stationary or moving slowly. These forces include both attractions and repulsions depending on the charge of the particles involved. Unlike ionic interactions that occur due to the transfer of electrons, electrostatic interactions can happen without any change in the electron configuration of the atoms or molecules involved. These interactions are pivotal in determining the physical and chemical properties of substances.
Comparison with Ionic Interactions
While ionic interactions result from the complete transfer of electrons between atoms, leading to the formation of charged ions, electrostatic interactions can involve not only fully charged ions but also partially charged atoms or molecules. This means electrostatic forces can be at play in a wider range of scenarios, affecting everything from the stability of compounds to the behavior of molecules in biological systems.
Types
Dipole-Dipole Interactions
These occur between molecules that have permanent dipole moments, meaning there is an uneven distribution of electron density within the molecule, leading to a positive end and a negative end. These permanent dipoles attract each other, stabilizing the structure of liquids and solids.
London Dispersion Forces
Even non-polar molecules can experience temporary dipoles due to momentary fluctuations in electron distribution. These fleeting dipoles induce similar dipoles in neighboring molecules, leading to London dispersion forces, a weak type of electrostatic interaction that is significant in non-polar substances.
Hydrogen Bonds
A special type of dipole-dipole interaction, hydrogen bonds, occur when a hydrogen atom covalently bonded to a highly electronegative atom like oxygen, nitrogen, or fluorine is attracted to another electronegative atom. Hydrogen bonds are stronger than typical dipole-dipole interactions and are crucial in the structure and function of water, proteins, and nucleic acids.
Role in Nature
Electrostatics in Water Solubility
Electrostatic interactions are fundamental in determining the solubility of substances in water. Ionic compounds dissolve in water as the polar water molecules interact with the ions, overcoming the ionic bonds. Similarly, polar molecules dissolve due to dipole-dipole interactions with water molecules.
Influence on Molecular Shape and Recognition
Electrostatic interactions influence the shape of molecules and how they recognize and bind to each other. For instance, the three-dimensional shape of proteins is partly determined by electrostatic forces, which also guide the binding of enzymes to their substrates or the interaction of antibodies with antigens.
Importance in Technology
Applications in Materials Science
In materials science, electrostatic interactions are harnessed to create novel materials with specific properties. For example, electrostatic forces can be used to assemble nanoparticles into complex structures or to create polymers with unique mechanical, electrical, or optical properties.
Electrostatics in Electrical Engineering
Electrostatic principles are the foundation of various electrical engineering applications, from capacitors, which store energy in an electric field, to electrostatic precipitators, which remove particles from exhaust gases using charged plates.
Comparison and Contrast
Key Differences Between Ionic and Electrostatic Interactions
The key difference lies in the nature of the forces involved. Ionic interactions are the result of the transfer of electrons and the formation of charged ions, leading to strong attractions that result in the formation of solid compounds. Electrostatic interactions, on the other hand, can involve both ions and neutral molecules and can manifest as both attractions and repulsions, influencing a broader range of physical and chemical processes.
Situational Examples Illustrating Their Distinct Roles
- Ionic interactions are crucial in the formation of salts, like sodium chloride, where the transfer of electrons from sodium to chlorine results in a solid with a high melting point.
- Electrostatic interactions are key in the behavior of water, where the polar nature of water molecules leads to hydrogen bonding, giving water its unique properties like high surface tension and the ability to dissolve many substances.
Influence on Molecular Behavior
How Each Interaction Affects Molecular Properties
Ionic interactions primarily affect the structural properties of compounds, giving rise to solids with high melting and boiling points. Electrostatic interactions, including dipole-dipole interactions and hydrogen bonding, influence the physical state, solubility, and boiling points of compounds.
Interaction with Water and Solubility
The solubility of substances in water is significantly influenced by electrostatic interactions. Ionic compounds dissolve as the water molecules disrupt the ionic bonds, and polar molecules dissolve due to their interactions with the polar water molecules. Non-polar molecules, lacking significant electrostatic interactions with water, are generally insoluble in water.
Energy Considerations
Comparison of Energy Levels in Ionic vs. Electrostatic Interactions
Ionic bonds typically involve greater energy changes during formation and dissociation than electrostatic interactions. The energy required to break ionic bonds is significantly higher, reflecting their role in forming stable compounds. Electrostatic interactions, while influential, involve lower energy changes, allowing for more dynamic molecular interactions and transformations.
Relevance to Chemical Reactions and Processes
Understanding the energy involved in ionic and electrostatic interactions is crucial in predicting the behavior of substances in chemical reactions and processes. For example, the solubility of ionic compounds in water can be influenced by the energy released when water molecules surround and interact with the ions.
Practical Applications
Ionic and Electrostatic Interactions in Drug Design
In drug design, both ionic and electrostatic interactions are considered to enhance the efficacy and specificity of drugs. For instance, designing drugs that form ionic bonds with their targets can lead to strong and specific interactions, while utilizing electrostatic interactions can improve the solubility and distribution of the drug within the body.
Role in Food Chemistry and Preservation
Electrostatic interactions play a role in the texture and stability of food products, influencing properties like emulsification and gelation. Ionic interactions are utilized in food preservation techniques, such as curing, where salt draws moisture out of foods through osmosis, inhibiting microbial growth.
Importance in Nanotechnology and Material Sciences
In nanotechnology, controlling ionic and electrostatic interactions allows for the assembly of nanomaterials with precise structures and functionalities. This has applications in creating new materials for electronics, catalysis, and biomedical devices.
Frequently Asked Questions
What are Ionic Interactions?
Ionic interactions are the forces that occur when electrons are transferred between atoms, leading to the formation of positively and negatively charged ions. These ions then attract each other to form ionic bonds, which are the basis of ionic compounds like salts. This type of interaction is essential for the structure and stability of many substances found in nature and used in various industrial applications.
How Do Electrostatic Interactions Differ from Ionic?
While ionic interactions involve the transfer of electrons and the formation of ionic bonds, electrostatic interactions refer to the forces of attraction and repulsion between particles with static charges, including ions, molecules, and atoms. These interactions are not limited to the formation of bonds but influence a wide range of phenomena, from the solubility of compounds to the behavior of molecules in biological systems.
Why are Ionic and Electrostatic Interactions Important in Biology?
In biology, ionic and electrostatic interactions are crucial for the structure, function, and regulation of biomolecules. Ionic interactions contribute to the stability of structures like proteins and DNA, affecting their formation and function. Electrostatic interactions, including hydrogen bonds and dipole-dipole forces, play key roles in molecular recognition, enzyme activity, and the dynamic behavior of cellular membranes.
Can Ionic and Electrostatic Interactions Influence Drug Design?
Yes, both ionic and electrostatic interactions are fundamental in drug design and development. Understanding these interactions allows scientists to predict how a drug will interact with its target, influencing its efficacy and specificity. By designing molecules that exploit specific ionic or electrostatic interactions, researchers can develop drugs with improved binding affinity, selectivity, and therapeutic effects.
Conclusion
The intricate ballet of ionic and electrostatic interactions underpins much of the physical and biological world, from the crystalline structure of salts to the complex folding of proteins. Recognizing and understanding these interactions not only enriches our grasp of chemistry and biology but also enhances our ability to manipulate matter for various applications, from materials science to pharmaceuticals. As we continue to uncover the nuances of these forces, we open new pathways to innovation and discovery, cementing their importance in the advancement of science and technology.
In the end, the journey through the realms of ionic and electrostatic interactions illuminates the fundamental principles that govern the natural world. It highlights the power of these interactions in shaping the very fabric of matter and life, offering insights that are crucial for both academic research and practical applications. As we delve deeper into their study, we unlock the potential to solve complex problems and create novel solutions that benefit humanity and the environment alike.