Redox reactions are fundamental to both natural processes and industrial applications, underscoring the need for accurate chemical equation balancing. These reactions, which involve the transfer of electrons between two species, are pivotal in fields ranging from biochemistry to environmental science. Balancing these equations ensures that the law of conservation of mass and charge is upheld, which is critical for the reaction to occur correctly.
The difference between the Ion-Electron Method and the Oxidation Number Method lies primarily in their approach to balancing redox reactions. The Ion-Electron Method, also known as the half-reaction method, separates the redox reaction into two half reactions—one for oxidation and one for reduction—each of which is balanced individually. The Oxidation Number Method, in contrast, focuses on changes in oxidation states of elements to balance the overall equation.
These two methods are indispensable tools in the chemistry toolkit, each with specific scenarios where they are most effective. Their correct application not only facilitates the understanding of the chemical processes involved but also ensures that the equations used in academic and practical settings are accurate and reliable.
Redox Reaction Basics
Definition and Examples
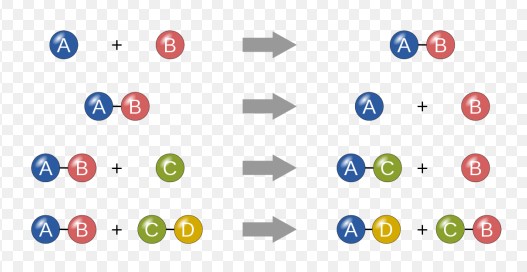
A redox reaction is a type of chemical process where oxidation and reduction simultaneously occur. Oxidation involves the loss of electrons, while reduction involves the gain of electrons. For example, in the reaction between hydrogen and oxygen to form water (2H₂ + O₂ → 2H₂O), oxygen is reduced as it gains electrons from hydrogen, which is oxidized.
Role in Chemical Equations
In chemical equations, redox reactions are essential for showing how electrons are transferred between substances. These reactions help scientists and chemists understand the energy changes and the reactivity of various chemicals. They also ensure that the equations adhere to the law of conservation of mass and charge.
Importance in Various Fields
Redox reactions are crucial across many fields:
- Environmental science: They play a role in biogeochemical cycles and pollution control.
- Energy production: Essential in batteries and fuel cells.
- Medicine: Involved in metabolic processes and drug delivery systems.
- Industrial manufacturing: Used in processes like the production of plastics and the refining of metals.
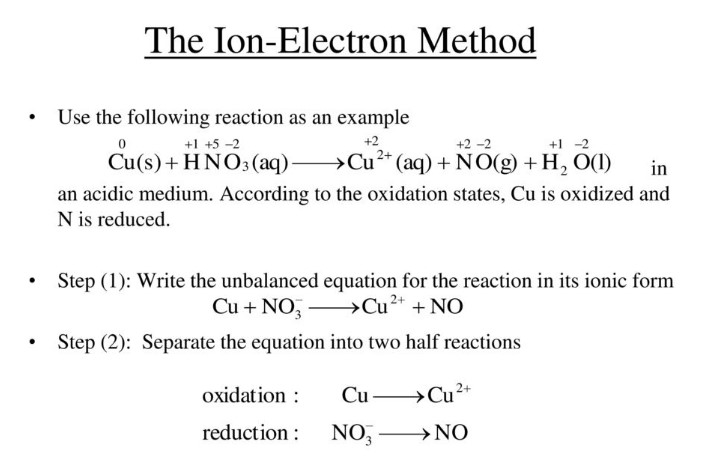
Ion-Electron Method
Concept Overview
The Ion-Electron Method, also known as the half-reaction method, is used to balance redox reactions by splitting them into two separate half-reactions. This method allows for the individual balancing of the oxidation and reduction processes.
Detailed Steps
Identifying Half Reactions
- Split the overall reaction into two parts: one for the oxidation reaction and one for the reduction reaction.
Balancing Atoms and Charges
- Balance all atoms except hydrogen and oxygen.
- Balance oxygen atoms by adding H₂O molecules.
- Balance hydrogen atoms by adding H⁺ ions (in acidic solutions) or OH⁻ ions (in basic solutions).
- Balance the charge by adding electrons.
Equalizing Electron Transfer
- Ensure the same number of electrons are lost in oxidation as are gained in reduction by multiplying the half-reactions by appropriate coefficients.
Applications and Examples
The Ion-Electron Method is widely used in:
- Environmental chemistry: For treating wastewater.
- Analytical chemistry: In determining the content of unknown samples.
- Educational purposes: Teaching students the balancing of complex redox reactions.
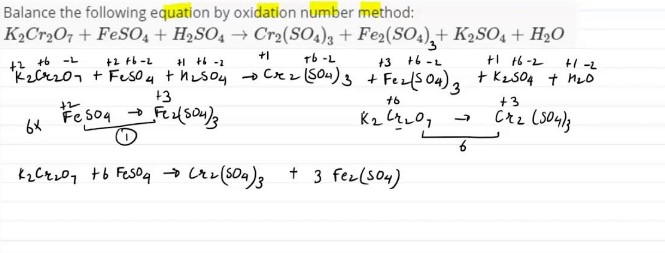
Oxidation Number Method
Concept Overview
The Oxidation Number Method involves balancing redox reactions based on the changes in oxidation numbers of the elements involved. It helps in quickly identifying the oxidizing and reducing agents in a reaction.
Detailed Steps
Assigning Oxidation Numbers
- Determine the oxidation number for each atom in the reactants and products.
Balancing Changes in Oxidation States
- Calculate the change in oxidation numbers for the elements undergoing redox changes.
- Use these changes to balance the redox reaction by ensuring that the increase in oxidation number (oxidation) is balanced by the decrease (reduction).
Using Coefficients to Balance the Equation
- Apply coefficients to the entire chemical equation to ensure that the number of atoms for each element is balanced on both sides of the equation.
Applications and Examples
The Oxidation Number Method is particularly useful in:
- Routine laboratory work: Quick balancing of straightforward equations.
- Educational settings: Helping students learn how to quickly determine the agents in redox reactions.
- Research: In studies where quick assessments of reactions are needed.
Comparison of Methods
Key Similarities
Both the Ion-Electron Method and the Oxidation Number Method are crucial for balancing redox reactions, which is essential in many scientific and industrial applications. Each method ensures that the chemical equation respects the conservation of mass and charge, and they both require a systematic approach to determine the electron flow in reactions.
Fundamental Differences
Conceptual Approach
The Ion-Electron Method focuses on separating the redox reaction into two half-reactions, each balanced individually. This method gives a clear picture of the electron transfer processes involved. On the other hand, the Oxidation Number Method works by tracking changes in oxidation states across the entire reaction, which often provides a quicker path to balancing.
Complexity and Suitability
The Ion-Electron Method can appear more complex because it involves multiple steps to balance the electrons, atoms, and charges separately in acidic or basic conditions. It is highly suited for complex redox reactions where differentiating between oxidation and reduction processes is necessary. The Oxidation Number Method is simpler and more straightforward, ideal for classroom settings or initial analyses where quick results are desired.
Accuracy and Preference in Use
Accuracy can vary between the two methods depending on the complexity of the reaction. The Ion-Electron Method is generally more accurate for complex reactions, while the Oxidation Number Method might suffice for simpler reactions. Preferences can vary among chemists depending on their familiarity with the methods and the specific requirements of the task.
Advantages of Each Method
Strengths of the Ion-Electron Method
- Detailed visualization of the reaction’s electron transfer
- Flexibility in handling complex reactions across different environments (acidic or basic)
- Educational value in teaching the step-by-step balancing of redox reactions
Strengths of the Oxidation Number Method
- Speed and simplicity in balancing equations
- Effectiveness in straightforward scenarios
- Ease of use for beginners and non-specialists
Limitations and Challenges
Challenges with the Ion-Electron Method
- It can be time-consuming to separate and balance the half-reactions correctly.
- Prone to errors in complex scenarios if steps are not followed meticulously.
- Requires a deeper understanding of the chemical behavior of the reactants and products.
Challenges with the Oxidation Number Method
- Less precise in complex chemical reactions where multiple oxidation states might confuse the balance.
- Limited application in educational settings where understanding the mechanics of electron transfer is crucial.
Decision Factors
Factors Influencing Method Choice
Type of Chemical Reaction
The nature of the reaction often dictates the method chosen. Complex organic reactions or those involving multiple electron transfers are better suited to the Ion-Electron Method. Simpler inorganic reactions may be more efficiently handled by the Oxidation Number Method.
Desired Accuracy
For research purposes or when exact quantification of substances is required, the Ion-Electron Method is often preferred due to its high accuracy. In contrast, when time is a constraint or the reaction is well-understood and straightforward, the Oxidation Number Method may be the better choice.
Contextual Application
In educational settings, the Oxidation Number Method can provide quick results for learning purposes, while the Ion-Electron Method might be reserved for more advanced studies or demonstrations. In industrial applications, the choice may depend on the process’s specific requirements, such as the need for precise balancing in chemical manufacturing.
Frequently Asked Questions
What is a Redox Reaction?
A redox reaction is a chemical process where oxidation and reduction occur simultaneously, resulting in the transfer of electrons between reactants. These reactions are crucial for numerous biological and industrial processes, such as cellular respiration and metal corrosion.
How Do You Identify a Redox Reaction?
You can identify a redox reaction by looking for changes in oxidation numbers in the reactants and products. A shift in these numbers indicates electron transfer, characteristic of redox processes.
What Makes the Ion-Electron Method Different?
The Ion-Electron Method breaks down the redox reaction into its constituent half-reactions, each representing either the loss or gain of electrons. This method allows for more straightforward balancing of complex reactions by focusing separately on the reduction and oxidation processes.
When Should You Use the Oxidation Number Method?
The Oxidation Number Method is best used when you need a quick and straightforward technique to balance equations where assigning oxidation states is relatively simple. It is especially useful in educational contexts or initial analyses.
Can These Methods Be Used Interchangeably?
While both methods aim to balance redox reactions, their use is not always interchangeable. The choice between them often depends on the complexity of the reaction and the preference or familiarity of the chemist or technician.
Conclusion
Balancing redox reactions accurately is crucial for scientific accuracy and practical applications, and both the Ion-Electron Method and Oxidation Number Method provide robust frameworks for achieving this. Each method offers unique advantages and is suited to different scenarios, reinforcing the importance of understanding their distinct approaches.
As we continue to explore and teach chemistry, appreciating these methods’ nuances not only enhances our comprehension but also empowers us to apply the right tools in our ongoing quest for scientific and technological advancement. Thus, a thorough grasp of these methods is essential for anyone involved in chemical sciences or related fields.