The intricate dance of electrons within an atom lays the foundation for the vast and varied world of chemistry. Two phenomena, the inert pair effect and the shielding effect, stand at the heart of understanding how elements behave and react with one another. While seemingly complex, these effects shed light on the nuances of atomic structure and its implications on chemical properties and reactivity.
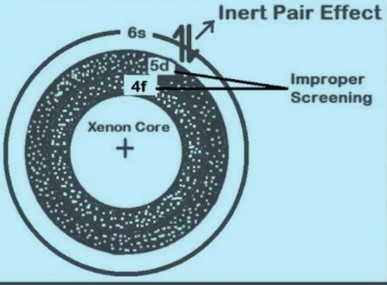
The inert pair effect refers to the tendency of the outermost s electrons in heavy atoms to remain non-bonding or inert, affecting the valency and chemical reactivity of elements, primarily seen in the p-block of the periodic table. On the other hand, the shielding effect describes how inner shell electrons can shield the outer electrons from the full charge of the nucleus, influencing an atom’s size, ionization energy, and electronegativity. Together, these effects provide critical insight into the behavior of atoms across the periodic table.
These concepts not only explain the reactivity patterns of elements but also guide scientists in predicting the outcomes of chemical reactions, designing new materials, and understanding the mechanisms of complex chemical processes. By examining the causes, examples, and impacts of the inert pair effect and shielding effect, one gains a deeper appreciation for the intricate details that govern the behavior of elements and compounds.
Inert Pair Effect
Definition
The inert pair effect is a phenomenon observed in heavy p-block elements, where the two electrons in the outermost s orbital (the “inert pair”) are less inclined to participate in chemical bonding. This reluctance affects the element’s valency and overall chemical reactivity.
Causes
Several factors contribute to the inert pair effect, primarily revolving around electron configuration and energy considerations. Key reasons include:
- Relativistic effects: As atomic number increases, the speed of electrons in s orbitals approaches a significant fraction of the speed of light, increasing their mass and energy. This makes them more stable and less likely to be involved in bonding.
- Effective nuclear charge: In heavy atoms, the inner electrons shield the outer electrons from the full charge of the nucleus. The s-electrons, being closer to the nucleus, are held more tightly than the p-electrons, making them less reactive.
- Energy stabilization: The energy required to promote these s-electrons into higher energy states for bonding is often not compensated by the energy released during bond formation, making it energetically unfavorable for them to participate.
Examples
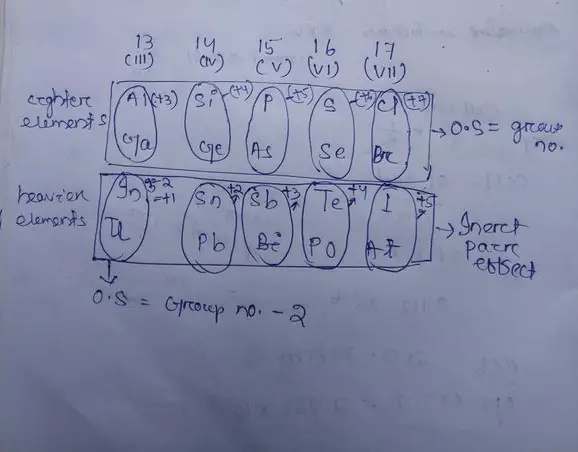
The inert pair effect is prominently seen in elements like lead (Pb) and thallium (Tl). For instance:
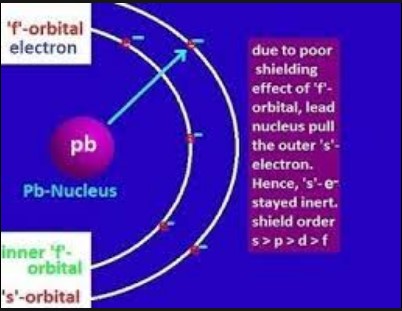
- Lead (Pb): Lead commonly exhibits an oxidation state of +2 instead of +4 due to the inert pair effect. This is evident in compounds like lead(II) oxide (PbO), where lead’s +2 state is stabilized.
- Thallium (Tl): Similarly, thallium prefers the +1 oxidation state over +3, as seen in thallium(I) sulfate (Tl2SO4), again due to the stabilization of the inert s-electrons.
Impact on Reactivity
The inert pair effect significantly influences the reactivity and valency of elements:
- Lower reactivity: Elements affected by the inert pair effect show reduced chemical reactivity, as their s-electrons are not readily available for bonding.
- Reduced valency: The effective valency of elements decreases, as seen in lead and thallium, affecting the types of compounds they can form.
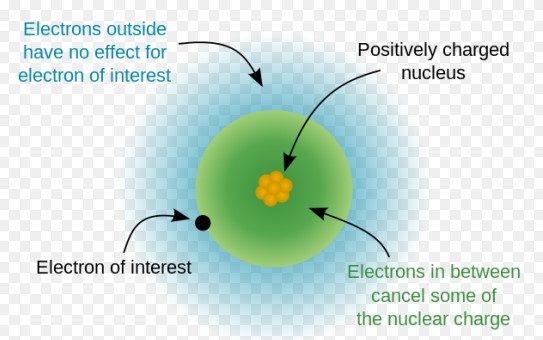
Shielding Effect
Definition
The shielding effect describes how electrons in inner shells reduce the force of attraction exerted by the nucleus on the outer electrons. This effect plays a crucial role in determining atomic sizes and ionization energies across the periodic table.
Causes
Key factors causing the shielding effect include:
- Electron-electron repulsion: Electrons in the same shell repel each other, reducing the effective nuclear charge felt by outer electrons.
- Orbital penetration: Electrons in s and p orbitals are closer to the nucleus and shield outer electrons more effectively than those in d and f orbitals.
Examples
Examples of the shielding effect are evident across the periodic table, especially in:
- Transition metals: The addition of electrons to the d orbital increases the shielding effect, affecting their atomic and ionic sizes.
- Lanthanides: The poor shielding effect of f-electrons leads to a decrease in atomic and ionic radii known as the lanthanide contraction.
Impact on Periodic Trends
The shielding effect influences key periodic trends:
- Atomic size: Increased shielding leads to larger atomic radii, as seen when moving down a group in the periodic table.
- Ionization energy: A stronger shielding effect results in lower ionization energies due to the reduced effective nuclear charge on outer electrons.
Comparative Analysis
Key Differences
The inert pair effect and shielding effect differ in several key aspects:
- Origin: The inert pair effect is due to the reluctance of s-electrons to participate in bonding, whereas the shielding effect involves the reduction of the nucleus’s effective charge by inner electrons.
- Affected elements: The inert pair effect is primarily observed in heavy p-block elements, while the shielding effect is universal across the periodic table.
- Consequences: The inert pair effect affects reactivity and valency, while the shielding effect influences atomic size and ionization energy.
Similarities
Despite their differences, both effects highlight the complex interplay of forces within an atom and underscore the importance of electron configuration in determining chemical properties.
Table Summary
A comparative summary:
| Feature | Inert Pair Effect | Shielding Effect |
|---|---|---|
| Definition | Reluctance of s-electrons to bond | Reduction of nucleus’s effective charge |
| Main Influence | Reactivity and valency | Atomic size and ionization energy |
| Affected Elements | Heavy p-block elements | All elements across the periodic table |
This table encapsulates the distinctions and the scope of influence each effect has on the chemical behavior of elements, providing a clear understanding of their roles in atomic chemistry.
Real-World Applications
Inert Pair Effect Applications
The inert pair effect has notable applications across various fields, from industrial processes to environmental science. By understanding how this effect influences the behavior of certain elements, scientists and engineers can harness it for innovative solutions.
Industrial Catalysts
One of the most significant applications of the inert pair effect is in the development of catalysts. For example, lead-based compounds, benefiting from the inert pair effect, are used in the synthesis of certain types of chemicals and fuels. These catalysts facilitate reactions at lower temperatures, reducing energy consumption and improving efficiency.
Medicine
In the pharmaceutical industry, the inert pair effect is exploited in the design of drug molecules. Elements showing this effect can form stable compounds that are used as active ingredients in medications, contributing to treatments for various diseases.
Environmental Chemistry
The inert pair effect also finds application in environmental remediation. Compounds stabilized by this effect are used to sequester heavy metals from waste streams, reducing their toxicity and environmental impact. For example, thallium-based compounds can be used to remove mercury from industrial wastewater.
Shielding Effect Applications
The shielding effect plays a crucial role in material science, nuclear chemistry, and other fields, underpinning technologies essential to modern life.
Material Science
In material science, understanding the shielding effect is crucial for designing alloys and semiconductors. It helps scientists predict how atoms will interact in a solid matrix, affecting the material’s electrical, magnetic, and mechanical properties. This knowledge is pivotal in developing new materials for electronics, aerospace, and automotive applications.
Nuclear Chemistry
The shielding effect is essential in nuclear chemistry for predicting the behavior of radioactive isotopes. It influences the penetration power of emitted radiation, which is critical for applications in medical imaging, cancer treatment, and nuclear power generation. By understanding how electrons shield the nucleus, chemists can manipulate isotopes to optimize their utility in these areas.
Challenges and Future Directions
Research Challenges
Despite the progress made in understanding the inert pair and shielding effects, challenges remain that hinder our full comprehension and exploitation of these phenomena.
Complex Interactions
One of the primary challenges is the complex interaction between electrons within atoms. Predicting how these interactions influence the inert pair and shielding effects requires sophisticated computational models and extensive experimental data, which are not always readily available.
Varying Conditions
The influence of these effects can vary significantly under different chemical and physical conditions, such as pressure, temperature, and the presence of other elements or compounds. This variability makes it difficult to generalize findings and apply them across different scenarios.
Future Prospects
The future of research in the inert pair and shielding effects is promising, with potential breakthroughs that could revolutionize chemical science.
Advanced Computational Models
Developments in quantum chemistry and computational methods are expected to provide more accurate predictions of these effects. These advancements could lead to the discovery of new materials with unprecedented properties and applications.
Innovative Applications
As our understanding deepens, novel applications for the inert pair and shielding effects are likely to emerge. In medicine, for example, this could mean more effective drug delivery systems or new treatments for diseases. In environmental science, it could lead to more efficient methods for pollution control and sustainable resource management.
Frequently Asked Questions
What is the Inert Pair Effect?
The inert pair effect is a phenomenon observed in heavy p-block elements where the s-electrons of the outermost shell exhibit a reluctance to participate in chemical bonding. This results in the element displaying a lower oxidation state than expected. It is particularly noticeable in elements like lead and thallium, where the inert s-electrons influence the chemical reactivity and stability of compounds formed by these elements.
How Does the Shielding Effect Influence Atomic Size?
The shielding effect impacts atomic size by determining the extent to which inner electrons can mitigate the nuclear charge experienced by the outermost electrons. A stronger shielding effect means that outer electrons feel less attraction to the nucleus, resulting in a larger atomic radius. This effect is crucial in understanding periodic trends, such as why atoms increase in size down a group in the periodic table.
Can the Inert Pair Effect be Predicted?
Predicting the inert pair effect involves understanding an element’s electron configuration and the relativistic effects impacting heavy atoms. While general trends can be anticipated, such as the effect being more pronounced in heavier p-block elements, precise predictions often require detailed quantum mechanical calculations that take into account the element’s environment and chemical state.
Why is the Shielding Effect Important in Chemistry?
The shielding effect is pivotal in chemistry for explaining variations in atomic and ionic radii, ionization energies, and electronegativity within the periodic table. It helps chemists predict how atoms will interact in molecules, the strength of these interactions, and the energy required to remove electrons. Understanding this effect is crucial for predicting reactivity patterns and tailoring chemical reactions for desired outcomes.
Conclusion
The exploration of the inert pair effect and the shielding effect unlocks a deeper understanding of chemical reactivity and the behavior of elements. These concepts not only elucidate the fundamental principles governing atomic interactions but also serve as a cornerstone for advancing chemical knowledge, from basic research to practical applications in various industries.
As we continue to uncover the mysteries of the atomic world, the insights gained from studying these effects will undoubtedly lead to new discoveries and innovations. The journey through the atomic landscape, guided by an understanding of the inert pair and shielding effects, remains a vital part of the ongoing quest to unravel the complexities of the chemical universe.