In the realm of organic chemistry, the distinction between imines and Schiff bases plays a pivotal role in the synthesis and applications of compounds. These functional groups, though closely related, exhibit unique characteristics that make them indispensable in various chemical reactions and product formulations. Understanding their differences not only enhances our grasp of organic synthesis but also opens the door to innovative applications in material science and catalysis.
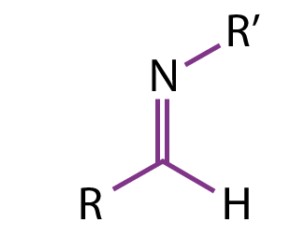
Imines are compounds formed by the condensation of primary amines with aldehydes or ketones, characterized by a carbon-nitrogen double bond. Schiff bases, a subset of imines, specifically arise from the reaction between an aldehyde and a primary amine. While both share a C=N bond, the specificity of the Schiff base’s formation process and its applications distinguish it from other imines.
The significance of these compounds extends beyond their definitions. Imines serve as intermediates in many organic reactions, including the synthesis of various alkaloids and pharmaceuticals. Schiff bases, known for their stability and versatile reactivity, are pivotal in catalysis and the creation of advanced materials. Their ability to form complexes with metals renders them invaluable in the field of coordination chemistry, further underscoring the breadth of their utility.
Basics of Imines
Definition and Structure
Imines are a class of organic compounds identified by a carbon-nitrogen double bond (C=N), where the nitrogen is connected to either a hydrogen atom or an alkyl or aryl group. This structural characteristic is essential to the imine’s reactivity and function in organic chemistry. The general formula for an imine can be represented as R1R2C=NR3, where R1 and R2 can be hydrogen, alkyl, or aryl groups, and R3 can be hydrogen or an alkyl group.
Formation Process
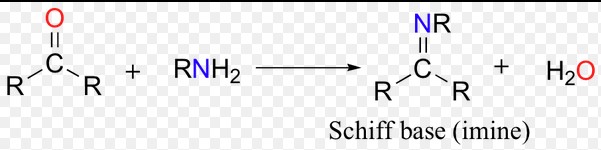
The formation of imines is a critical reaction in organic synthesis, known as the condensation reaction. This process involves two main steps:
- Reaction of a primary amine with an aldehyde or ketone: This step leads to the formation of an intermediate compound.
- Dehydration: The intermediate then undergoes dehydration (loss of water) to form the imine.
This process is important because it introduces a way to form bonds between carbon and nitrogen, a key connection in many biological and synthetic compounds.
Properties
Imines exhibit a range of physical and chemical properties that make them valuable in organic chemistry. Physically, they can vary from being volatile liquids to crystalline solids, depending on the nature of the substituents attached to the nitrogen atom. Chemically, imines are relatively reactive, especially towards nucleophiles, due to the polar nature of the carbon-nitrogen double bond. This reactivity is exploited in numerous synthetic applications, including the formation of amines and the synthesis of various heterocycles.
Basics of Schiff Bases
Definition and Structure
Schiff bases are a subset of imines, where the carbon-nitrogen double bond is formed between an aldehyde and a primary amine. The distinction lies in the Schiff base’s specific formation from the aldehyde, which introduces a unique set of properties to these compounds. The general structure of a Schiff base can be represented as R1-CH=N-R2, highlighting the linkage formed from the aldehyde component.
Formation Process
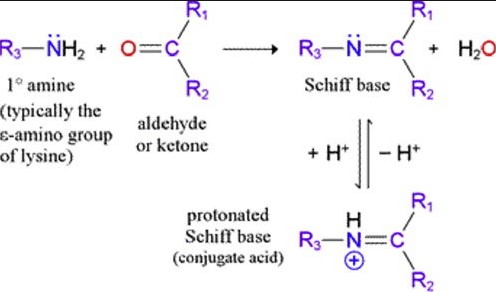
The formation of Schiff bases involves a condensation reaction similar to that of general imines but with specific reactants. The steps include:
- Combining an aldehyde with a primary amine: This leads to the initial formation of a Hemiaminal, a compound with both OH and NH2 groups attached to the same carbon.
- Dehydration: The Hemiaminal then loses a molecule of water to form the Schiff base.
This condensation reaction is not only simple but also versatile, allowing for the synthesis of a wide range of compounds with diverse applications.
Properties
Schiff bases are known for their unique properties, including:
- Stability: Compared to other imines, Schiff bases are generally more stable, which makes them useful in applications that require long-term storage or stability under reaction conditions.
- Reactivity: Despite their stability, Schiff bases are reactive towards certain reagents, especially metal ions. This reactivity is crucial in the formation of metal complexes, making Schiff bases important in catalysis and material science.
Key Differences Between Imines and Schiff Bases
Chemical Structure
The chemical structures of imines and Schiff bases are similar at a glance, both featuring a carbon-nitrogen double bond. However, a closer look reveals distinct differences.
- Imines can be generalized by the structure R1R2C=NR3, where R1 and R2 can be hydrogen, alkyl, or aryl groups, and R3 is either hydrogen or another alkyl or aryl group. This broad definition allows for a variety of compounds within the imine category.
- Schiff bases, on the other hand, have a more specific structure, with one of the groups attached to the carbon being a result of an aldehyde (R1-CH=N-R2). This specificity not only influences their formation but also their applications and reactivity.
Formation Mechanism
The formation mechanisms of imines and Schiff bases showcase their distinctive paths.
- Imines are formed through the condensation of primary amines with aldehydes or ketones, followed by dehydration. This process can occur with a variety of carbonyl compounds, making it versatile.
- Schiff bases form specifically through the reaction between aldehydes and primary amines. The distinction here is not just the specificity of the carbonyl compound (aldehyde) but also the resultant stability and application that differ due to the nature of the starting materials.
Stability and Reactivity
Stability and reactivity are crucial properties that differentiate imines from Schiff bases.
- Imines tend to be less stable than Schiff bases, partially due to their broader variety of structures and the ease with which they can hydrolyze back to the original aldehyde or ketone and amine.
- Schiff bases, characterized by their stability, are less prone to hydrolysis. Their stability is a key feature that enables their widespread use in applications requiring durable compounds. Moreover, Schiff bases’ reactivity, particularly in forming metal complexes, is a valuable asset in catalysis and material science.
Applications
When comparing applications, both imines and Schiff bases contribute significantly to various fields, albeit in different ways.
- Imines are often used as intermediates in organic synthesis, including the synthesis of amines, alkaloids, and pharmaceuticals. Their reactivity makes them suitable for a range of chemical transformations.
- Schiff bases have found a niche in catalysis and material science. Their ability to form stable complexes with metals makes them invaluable in the synthesis of polymers, catalysts, and advanced materials.
Applications and Significance
In Organic Synthesis
The role of imines and Schiff bases in organic synthesis cannot be overstated. They are pivotal as intermediates and reactants in the synthesis of complex organic compounds.
- Imines are utilized in the synthesis of alkaloids, pharmaceuticals, and amines through various organic reactions, including reductive amination and the Mannich reaction.
- Schiff bases, thanks to their stability, are frequently employed in synthetic pathways to produce complex molecules, serving as precursors to many pharmaceuticals and organic materials.
In Catalysis
Both imines and Schiff bases play a critical role in catalysis, facilitating reactions that would otherwise be inefficient or impossible.
- Imines act as catalysts or intermediates in catalytic cycles, especially in the formation of new carbon-nitrogen bonds. Their versatility and reactivity enable the catalysis of a wide range of transformations.
- Schiff bases are particularly valued in metal-catalyzed reactions. They form complexes with metals that are catalysts in processes such as hydrogenation, oxidation, and carbon-carbon bond formation. This ability to stabilize metal ions extends their application to environmental catalysis, fine chemical synthesis, and beyond.
In Material Science
Schiff bases stand out in the development of advanced materials. Their metal complexes find applications in:
- Optical materials: Utilizing their ability to form complexes with metals, Schiff bases contribute to the development of materials with unique optical properties.
- Magnetic materials: Certain Schiff base metal complexes exhibit magnetic properties, useful in data storage technologies.
- Sensors and detectors: The specific reactivity of Schiff bases with metals can be harnessed to develop sensors for detecting various ions and molecules.
Frequently Asked Questions
What is an Imine?
An imine is an organic compound characterized by a carbon-nitrogen double bond, where the nitrogen atom is connected to a hydrogen atom or an alkyl or aryl group. These compounds are crucial intermediates in organic synthesis, serving as platforms for the construction of more complex molecules.
How is a Schiff Base Different from an Imine?
A Schiff base is a specific type of imine formed exclusively from the condensation of an aldehyde with a primary amine. What sets Schiff bases apart is their unique stability and their widespread use in catalysis and material science, especially in forming complexes with metals.
Can Imines and Schiff Bases Act as Catalysts?
Yes, both imines and Schiff bases can act as catalysts in a variety of chemical reactions. Their ability to form stable complexes with metals allows them to facilitate reactions in organic synthesis and polymerization processes, highlighting their versatility and importance in catalysis.
What Are the Applications of Schiff Bases?
Schiff bases have extensive applications in organic chemistry, material science, and catalysis. They are particularly notable for their role in the synthesis of pharmaceuticals, polymers, and as ligands in coordination chemistry, where they form complexes with metals for catalytic and analytical purposes.
Conclusion
The exploration of imines and Schiff bases reveals a rich landscape of chemistry where functionality and application converge. These compounds not only serve as fundamental building blocks in the synthesis of complex molecules but also as key players in the development of new materials and catalytic processes. Their distinct properties and applications underscore the importance of understanding these functional groups in the broader context of chemical science.
Recognizing the differences and similarities between imines and Schiff bases enriches our appreciation for organic chemistry’s nuances. As we continue to discover their roles in various domains, from pharmaceuticals to material science, the knowledge of these compounds will remain a cornerstone in the advancement of chemical research and innovation.