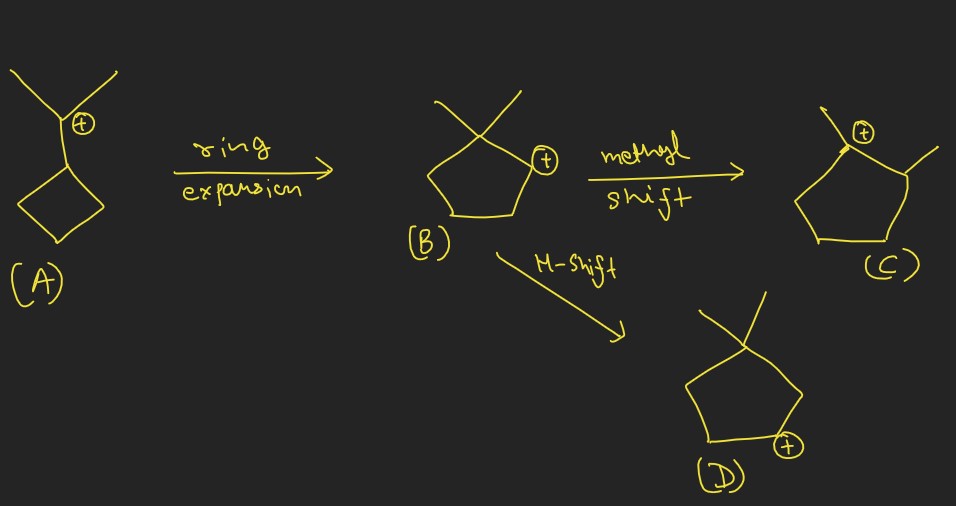
In the realm of organic chemistry, the subtle movements of atoms within molecules during chemical reactions pave the way for complex transformations. Among these movements, hydride and methyl shifts stand out as crucial mechanisms that significantly alter the structure and properties of organic compounds. These shifts are not merely steps in a reaction but pivotal moments that can dictate the course and outcome of synthetic pathways.
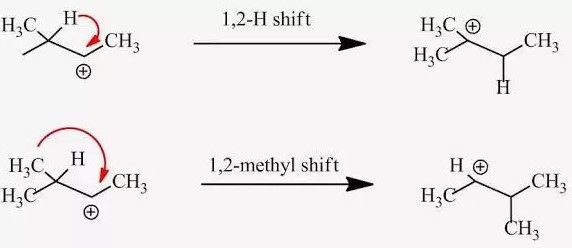
The difference between a hydride and a methyl shift lies in the type of moving group: a hydride shift involves the transfer of a hydrogen atom with its accompanying electrons, while a methyl shift pertains to the movement of a methyl group. These shifts occur under specific conditions and are instrumental in stabilizing carbocations during reactions, leading to the successful formation of desired products.
Hydride and methyl shifts play pivotal roles in rearrangement reactions, influencing the stability and reactivity of intermediates. By facilitating the migration of atoms or groups within a molecule, they provide a pathway to more stable carbocation configurations, enabling the synthesis of complex molecules with high precision. Understanding these shifts is essential for chemists looking to harness the full potential of organic synthesis and tailor reactions for optimal outcomes.
Basics of Shifts
What is a Shift?
In the world of organic chemistry, a shift is akin to a strategic move in a chess game. It’s when atoms or groups of atoms within a molecule relocate from one position to another. This relocation plays a critical role in chemical reactions, influencing both the process and the products formed. Shifts are not just random movements; they are guided by the need for stability and the quest for the most favorable energy state of a molecule.
Types of Shifts
Shifts in chemistry come in various forms, each with its unique significance and implications. The most common types include:
- Hydride Shifts: Involves the movement of a hydride ion (a hydrogen atom with an extra electron, �−H−).
- Methyl Shifts: Entails the transfer of a methyl group (��3CH3) from one carbon atom to another.
- Alkyl Shifts: Similar to methyl shifts but involves alkyl groups (larger carbon groups than methyl) moving within the molecule.
- Ring Expansions and Contractions: Occur when carbon rings increase or decrease in size through atom shifts.
Each of these shifts can dramatically change a molecule’s structure and, consequently, its reactivity and properties.
Hydride Shift
What is Hydride?
Hydride refers to a hydrogen atom that has gained an extra electron, resulting in a negative charge (�−H−). This ion is crucial in organic reactions, particularly in shifts where its movement can stabilize carbocations, intermediates prone to rearrangement due to their positive charge.
Mechanism of Hydride Shift
The hydride shift mechanism is fascinating and occurs under specific conditions:
- Presence of a Carbocation: A positively charged carbon atom in the molecule is a prerequisite for a hydride shift.
- Need for Stability: The shift tends to occur when it can result in a more stable carbocation configuration.
Steps in a Hydride Shift:
- Formation of Carbocation: The reaction begins with the formation of a carbocation, a step that can result from various processes like the loss of a leaving group.
- Movement of Hydride: The hydride ion adjacent to the carbocation then moves to the positively charged carbon.
- Formation of New Carbocation: This shift results in a new carbocation, ideally in a position that is more stabilized by the molecular surroundings.
Example Reactions:
- Pinacol Rearrangement: In this reaction, a diol undergoes acid-mediated dehydration, leading to a carbocation that stabilizes through a hydride shift, eventually forming a ketone.
- Wagner-Meerwein Rearrangement: An alkyl group migrates from one carbon to a neighboring carbocation, which can involve hydride shifts for stabilization.
Methyl Shift
What is Methyl?
Methyl groups (��3CH3) are among the simplest alkyl groups in organic chemistry. Despite their simplicity, they play a significant role in molecular rearrangements, including methyl shifts, where a methyl group moves from one carbon atom to another within a molecule.
Mechanism of Methyl Shift
Methyl shifts share similarities with hydride shifts in terms of the conditions under which they occur but differ in the moving group:
- Presence of a Carbocation: A carbocation is also a prerequisite for methyl shifts.
- Stabilization Goal: The shift aims to achieve a more stable carbocation configuration through the relocation of a methyl group.
Steps in a Methyl Shift:
- Formation of Carbocation: The process begins with carbocation formation, often through similar mechanisms as in hydride shifts.
- Movement of Methyl Group: A methyl group adjacent to the carbocation then migrates towards it, attaching to the positively charged carbon.
- New Carbocation Configuration: This results in a new carbocation, again in a more stable position due to the molecular environment.
Example Reactions:
- Neopentyl Rearrangement: A classic example where a methyl shift occurs during the reaction of neopentyl derivatives, leading to more stable carbocation intermediates.
- Beckmann Rearrangement: Although primarily known for transforming ketoximes into amides, certain conditions can induce methyl shifts that affect the rearrangement outcome.
Comparing Shifts
Key Differences
When exploring hydride and methyl shifts, it’s crucial to understand their distinctions, which primarily lie in their chemical properties and reaction conditions.
- Chemical Properties: A hydride shift involves the movement of a hydrogen atom with its two electrons, leading to a negative charge distribution. In contrast, a methyl shift involves a methyl group (��3CH3), which moves as a neutral entity. The nature of the moving group significantly influences the reaction’s mechanics and the intermediate’s stability.
- Reaction Conditions: Hydride shifts typically occur in environments where electron-rich hydrogen can stabilize a positively charged carbon. Methyl shifts, however, are more likely in scenarios where a larger alkyl group can better distribute the positive charge across the molecule.
Similarities
Despite their differences, hydride and methyl shifts share important roles in chemical rearrangements:
- Both shifts are mechanisms for molecule stabilization during reactions, aiming to distribute charges or create more stable carbocations.
- They can influence reaction pathways and outcomes, often leading to unexpected but beneficial products.
Factors Influencing Shifts
Stability Considerations
Carbocation stability is a paramount factor affecting shifts:
- The formation and stabilization of carbocations are pivotal. A shift, whether hydride or methyl, is usually observed when it results in a more stable carbocation, adhering to the order of carbocation stability (��������>���������>�������tertiary>secondary>primary).
Steric Effects
The size and shape of molecules play a significant role in determining shift preference:
- Steric hindrance, or the repulsion between electron clouds of atoms in close proximity, can influence whether a hydride or methyl shift is more favorable. Larger groups might prefer pathways that minimize these repulsions.
Reaction Conditions
Various conditions can sway the type of shift observed:
- Solvent, temperature, and presence of catalysts can all alter the course of a reaction, making one type of shift more favorable over another due to changes in reaction speed, stability, or mechanism.
Practical Implications
Synthesis Applications
Understanding and leveraging hydride and methyl shifts have profound implications in organic synthesis:
- Synthetic Routes: Knowledge of these shifts allows chemists to design synthetic pathways that can yield complex molecules with high specificity and efficiency.
- Reaction Optimization: By controlling reaction conditions to favor one type of shift over another, chemists can optimize yields and selectivity for desired products.
Impact on Product Formation
The type of shift occurring during a reaction can significantly influence the final product:
- Structural Isomers: Shifts can lead to different structural isomers, affecting the molecule’s properties and utility.
- Reaction Selectivity: The specificity of a shift can determine the selectivity of a reaction, crucial for producing a single product from a set of possible outcomes.
Frequently Asked Questions
What triggers a hydride or methyl shift?
Hydride and methyl shifts are typically triggered by the formation of an unstable carbocation intermediate during a reaction. The molecule rearranges itself to achieve a more stable carbocation state, with the shift occurring to minimize energy and increase stability.
Can both shifts occur in one reaction?
Yes, both hydride and methyl shifts can occur in a single reaction, depending on the structure of the reacting molecule and the nature of the intermediates formed. The sequence and occurrence of these shifts are dictated by the need to achieve the most stable carbocation configuration.
How do shifts affect reaction outcomes?
Shifts directly influence the structural outcome of a reaction by determining the position and connectivity of atoms in the final product. By altering the course of intermediate transformations, they can lead to different products from what might be expected without such rearrangements.
Why are these shifts important in organic synthesis?
Hydride and methyl shifts are vital in organic synthesis because they allow for the rearrangement of molecules in a way that can significantly enhance the stability and reactivity of reaction intermediates. This enables the synthesis of complex molecules and the development of more efficient and selective synthetic routes.
Conclusion
The intricate dance of atoms during hydride and methyl shifts exemplifies the beauty and complexity of organic chemistry. These shifts not only highlight the dynamic nature of chemical reactions but also underscore the importance of understanding molecular rearrangements for the synthesis of complex organic compounds. Through the lens of these shifts, chemists can unravel the mysteries of molecular transformation, paving the way for advances in drug development, materials science, and beyond.
As we continue to explore the depths of organic chemistry, the knowledge of hydride and methyl shifts remains a beacon, guiding the way toward innovative solutions and discoveries. By mastering the nuances of these mechanisms, scientists and researchers are equipped to push the boundaries of what is possible in synthetic chemistry, contributing to the progress of numerous fields reliant on organic compounds.