The distinction between hydration and hydrogenation may seem subtle at first glance, yet it holds significant importance across various industries, from pharmaceuticals to food production. Each process, involving the addition of water or hydrogen respectively, plays a pivotal role in altering the chemical structure and properties of compounds. This alteration not only impacts the physical state of a substance but also its application and effectiveness in its intended use.
Hydration involves the addition of water to a compound, a fundamental process in many biological and chemical reactions essential for life and industrial applications. Hydrogenation, on the other hand, involves the addition of hydrogen to molecules, often altering unsaturated bonds to saturated ones, impacting their stability, melting point, and physical appearance. This process is crucial in creating products ranging from margarine to biodiesel.
These processes are cornerstone techniques in chemical engineering and chemistry, each with its unique catalysts, methodologies, and implications. Hydration can increase solubility or change the reactivity of a compound, while hydrogenation can transform liquids into solids, such as oils into fats, making them integral to product formulation and innovation in various sectors.
Core Concepts
What is Hydration?
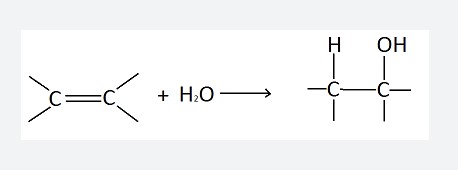
Hydration is a chemical process that involves the addition of water (H2O) to another compound. It’s a critical reaction in both natural and industrial contexts, affecting how substances interact and change. When a compound undergoes hydration, its structure incorporates water molecules, which can significantly alter its properties. This process is pivotal in various applications, from creating construction materials like concrete, which hardens through hydration, to enabling biochemical reactions within living organisms.
What is Hydrogenation?
Hydrogenation refers to the chemical addition of hydrogen (H2) to another molecule, often in the presence of a catalyst like nickel or palladium. This process is essential in transforming the physical and chemical properties of substances. For instance, hydrogenation can convert liquid vegetable oils into solid or semi-solid fats, a transformation crucial for food processing industries. The presence of a catalyst is necessary to lower the activation energy of the reaction, allowing hydrogen atoms to bond with the compound’s molecules more efficiently.
Key Differences
Chemical Process
The core difference between hydration and hydrogenation lies in their chemical processes. Hydration adds water molecules to a compound, often impacting its solubility and reactivity. In contrast, hydrogenation involves the addition of hydrogen atoms, affecting the compound’s saturation level and stability. While hydration can lead to the formation of new compounds or ions, hydrogenation typically alters the bonding structure within molecules, such as converting unsaturated fats to saturated fats.
Catalysts Used
Catalysts play a crucial role in both processes but differ in their nature and application:
- Hydration reactions sometimes occur spontaneously or can be facilitated by acids or bases as catalysts.
- Hydrogenation requires metal catalysts, such as nickel, palladium, or platinum, to proceed at a practical rate and temperature.
These catalysts are essential for lowering the energy barrier of the reactions, making them more efficient and viable for industrial applications.
Industry Applications
Both hydration and hydrogenation have wide-ranging applications across different industries, significantly impacting product properties:
- Hydration is fundamental in the construction industry for creating concrete and plaster. It’s also crucial in the pharmaceutical and food industries, where hydration reactions are used to improve the solubility and absorption of compounds.
- Hydrogenation is predominantly used in the food industry to produce solid fats from liquid oils, creating products like margarine and shortening. It also plays a role in the chemical industry, where hydrogenation is used to produce synthetic rubbers and fuel additives.
End Products
The end products of hydration and hydrogenation processes vary, reflecting the changes in physical and chemical properties:
- Hydrated compounds can become more reactive or soluble, which is essential for various industrial processes and products, such as pharmaceuticals and construction materials.
- Hydrogenated compounds often become more stable and solid, as seen in the production of solid fats for the food industry and in creating longer-lasting chemical products.
Detailed Insights
Health Impacts
The health impacts of these processes are significant, particularly in the context of hydrogenated products:
- Trans fats, a result of partial hydrogenation of vegetable oils, have been linked to various health issues, including heart disease. The process alters the natural cis configuration of fat molecules to a trans configuration, affecting their metabolic processing.
- Hydrated compounds, on the other hand, can play beneficial roles, such as hydration reactions in the body facilitating nutrient absorption and metabolic processes.
Environmental Considerations
The environmental impact of hydration and hydrogenation varies:
- Hydration reactions, especially those occurring naturally or in benign industrial applications, tend to have minimal negative environmental impact.
- Hydrogenation processes, particularly those requiring high energy input and involving metal catalysts, can have a more substantial environmental footprint. Advances in catalyst design and reaction efficiency aim to reduce these impacts.
Technological Advances
Recent technological advances have improved both hydration and hydrogenation processes:
- Innovations in catalyst technology have made hydrogenation reactions more efficient and environmentally friendly. For example, the development of nano-catalysts offers higher reactivity with lower amounts of catalyst and waste.
- Technological improvements in hydration processes have focused on enhancing efficiency and product quality, such as using supercritical water to increase reaction rates and selectivity.
Practical Applications
In Food Industry
Hydration and hydrogenation are cornerstone processes in food production and preservation, each serving distinct roles to enhance the quality, stability, and appeal of food products.
- Hydration is vital in processes like dough formation where water interacts with flour to develop gluten, essential for the texture of baked goods. In beverage production, hydration reactions are crucial for dissolving flavors, vitamins, and minerals, enhancing the nutritional profile and taste of drinks.
- Hydrogenation plays a key role in increasing the shelf life and stability of oils, preventing them from becoming rancid. It converts unsaturated fats into saturated fats, making oils solid or semi-solid at room temperature. This process is fundamental in the production of margarine, shortening, and certain snack foods, contributing to their creamy texture and resistance to spoilage.
In Cosmetics
Both hydration and hydrogenation processes significantly impact the formulation and effectiveness of cosmetic products.
- Hydration is essential in creating products like moisturizers and serums, where water acts as a solvent, delivering active ingredients to the skin. It’s also crucial in hydrogel production, used in masks and hydrating patches, offering deep hydration and a cooling effect.
- Hydrogenation contributes to the stability and texture of products such as lipsticks and creams. By transforming liquid oils into solid forms, it allows for the development of long-lasting, smooth, and spreadable cosmetic products. Hydrogenated oils are also used as emollients, softening and moisturizing the skin by forming a protective barrier.
In Pharmaceuticals
The pharmaceutical industry relies heavily on hydration and hydrogenation for drug development and manufacturing, affecting drug solubility, efficacy, and stability.
- Hydration can improve the solubility of active pharmaceutical ingredients (APIs), enhancing their bioavailability and absorption in the body. This is crucial for the effectiveness of oral medications and injectables.
- Hydrogenation is employed to modify the structure of APIs, potentially reducing toxicity, enhancing stability, and improving pharmacokinetic properties. This process can be key to the success of certain drugs, influencing their therapeutic effect and shelf life.
Economic Impact
Cost Implications
The cost implications of hydration and hydrogenation processes can vary significantly based on the scale of operation, the specificity of the catalysts used, and the energy requirements.
- Hydration processes generally involve lower costs, especially when the reactions occur naturally or under mild conditions. The use of water, an abundant and inexpensive solvent, helps minimize expenses. However, specialized equipment or catalysts required for certain hydration reactions can increase costs.
- Hydrogenation, on the other hand, often incurs higher costs due to the need for specific metal catalysts and high-pressure equipment. The cost of hydrogen gas and the maintenance of safe operating conditions also contribute to the overall expenses. However, the value added by hydrogenation, such as extended shelf life and improved product stability, can justify these costs in many industries.
Market Trends
The market demand for hydrogenated and hydrated products reflects evolving consumer preferences and regulatory trends.
- The demand for hydrogenated foods has seen fluctuations due to health concerns over trans fats. However, there’s still significant demand in markets that value product stability and shelf life, like in certain baked goods and snack foods. Innovations in full or selective hydrogenation that minimize health risks while preserving product qualities are influencing market trends.
- Hydrated products, especially in the cosmetics and pharmaceutical sectors, continue to see strong demand due to the growing consumer interest in health and wellness. Products promoting hydration benefits, such as moisturizers containing hyaluronic acid or isotonic drinks that quickly replenish body fluids, are increasingly popular.
- The future outlook suggests a continued interest in both hydrogenated and hydrated products, with a strong emphasis on sustainability, health, and technological advancements to improve efficiency and reduce environmental impact.
Frequently Asked Questions
What is hydration in chemistry?
Hydration in chemistry refers to the process of adding water molecules to a substance. It plays a critical role in various biological and chemical reactions, affecting the solubility, reactivity, and physical properties of compounds. This process is essential in fields ranging from pharmacology to food science, where it impacts the effectiveness and characteristics of products.
How does hydrogenation affect food?
Hydrogenation transforms unsaturated fats into saturated fats, impacting the texture, shelf life, and melting point of food products. It’s widely used in the food industry to convert oils into semi-solid forms, creating margarine and shortening, which enhances the stability and palatability of processed foods.
Why are catalysts used in hydrogenation?
Catalysts are used in hydrogenation to increase the efficiency and speed of the chemical reaction. They enable the hydrogen atoms to bond more readily to the unsaturated bonds of molecules, without being consumed in the process. Common catalysts include metals such as palladium, nickel, and platinum, which are crucial for facilitating this transformation in an economically viable manner.
What are the environmental impacts of hydrogenation?
The environmental impacts of hydrogenation include energy consumption and potential pollution from byproducts. While the process is essential for creating various products, it requires significant energy, and the use of certain catalysts can lead to environmental concerns. Advances in green chemistry aim to mitigate these effects through more sustainable practices and materials.
Conclusion
The intricate dance between hydration and hydrogenation underscores their pivotal role in shaping the properties of countless products that touch our everyday lives. From the creams that moisturize our skin to the foods that nourish our bodies, these chemical processes influence texture, stability, and functionality. Understanding their differences and applications not only demystifies the science behind our daily products but also highlights the ingenuity at play in creating them.
As industries continue to evolve, the significance of hydration and hydrogenation processes in product development and innovation cannot be overstated. The continued research and development in these areas promise to enhance the efficacy, sustainability, and environmental impact of future products, underscoring the importance of chemical processes in advancing technology and improving quality of life.