Aromatic compounds, the cornerstone of organic chemistry, are categorized based on their molecular structures and the nature of the atoms within their rings. These compounds are not only fundamental in chemistry but also pivotal in numerous scientific and industrial applications. At the core, the distinction between homonuclear and heteronuclear aromatic compounds lies in the types of atoms composing their cyclic structures.
Homonuclear aromatic compounds consist solely of one type of atom, typically carbon, as seen in benzene. On the other hand, heteronuclear aromatic compounds include different types of atoms, such as nitrogen in pyridine. This fundamental difference dictates their chemical behavior, physical properties, and applications, making it essential to distinguish between these two types.
The exploration of these compounds reveals intricate details about their stability, reactivity, and usability in various fields. From pharmaceuticals to materials science, understanding the differences between homonuclear and heteronuclear aromatic compounds helps in tailoring them for specific purposes and enhancing their efficacy in desired applications.
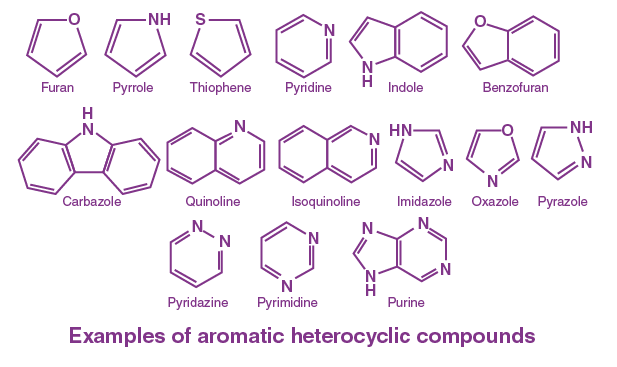
Aromatic Compounds Basics
Definition and Characteristics
Aromatic compounds are a distinctive class of organic molecules known for their highly stable ring structures. These rings contain alternating double and single bonds arranged in a conjugated system, typically involving pi-electrons shared across the structure. The most notable characteristic of aromatic compounds is their extraordinary chemical stability, which is a result of electron delocalization providing significant energetic stability to the molecule. This stability, termed aromaticity, is not only a defining trait but also influences how these compounds react with other substances.
Role in Chemistry and Applications
Aromatic compounds play a pivotal role in modern chemistry. Their unique properties make them essential in the synthesis of a vast array of chemical products, ranging from pharmaceuticals to dyes and polymers. For example, benzene, a simple aromatic compound, is foundational in manufacturing plastics, resins, and synthetic fibers. Moreover, their predictable stability and reactivity patterns make aromatic compounds ideal candidates for organic synthesis in laboratory settings and industrial applications.
Homonuclear Aromatic Compounds
Definition and Examples
Homonuclear aromatic compounds are those that consist of a ring primarily made up of one type of atom, usually carbon. The quintessential example of a homonuclear aromatic compound is benzene, symbolized by its hexagonal ring and alternating double bonds. Other examples include naphthalene and anthracene, both of which are composed solely of carbon and hydrogen atoms and are used extensively in the production of dyes and pigments.
Structure and Properties
The structure of homonuclear aromatic compounds contributes directly to their notable properties such as thermal stability and electrical conductivity. These compounds possess a planar ring structure that allows for the electrons to be delocalized over the entire ring, providing significant chemical resilience and lower reactivity compared to non-aromatic compounds. Their unique electronic configuration also makes them useful in applications requiring conductive materials.
Common Uses and Significance
The significance of homonuclear aromatic compounds extends beyond the laboratory. They are fundamental in the production of a variety of everyday materials. For instance, benzene derivatives are crucial in the manufacture of nylon and synthetic rubbers. The predictability and stability of these compounds ensure they are invaluable in creating consistent and durable products.
Heteronuclear Aromatic Compounds
Definition and Examples
In contrast to homonuclear compounds, heteronuclear aromatic compounds include heteroatoms such as nitrogen, oxygen, or sulfur within their cyclic structures. Pyridine, with a nitrogen atom replacing one of the carbon atoms in benzene, serves as a primary example. Other examples include furan (oxygen as the heteroatom) and thiophene (sulfur as the heteroatom), each exhibiting unique properties due to the presence of these heteroatoms.
Structure and Properties
The introduction of heteroatoms changes the electronic structure of these compounds. Heteronuclear aromatic compounds often display differences in electronegativity, altering their physical and chemical properties. For instance, the presence of a nitrogen atom in pyridine increases its basicity compared to benzene. These structural differences impact their solubility, boiling points, and overall reactivity.
Importance in Various Fields
Heteronuclear aromatic compounds are crucial in fields such as pharmaceuticals, where their variability and functional diversity allow for the synthesis of complex molecules. Pyridine derivatives are commonly used in drugs that treat various ailments, showcasing their versatility and crucial role in medicinal chemistry.
Key Differences
Atomic Composition
The fundamental difference between homonuclear and heteronuclear aromatic compounds lies in their atomic composition. Homonuclear compounds consist of only one type of atom in their rings, primarily carbon, whereas heteronuclear compounds incorporate different atoms, introducing diversity in their chemical makeup.
Chemical Properties
This variation in composition leads to distinct chemical properties. Heteronuclear compounds often exhibit varied reactivity and selectivity due to the presence of heteroatoms, which can engage in additional types of chemical reactions compared to their homonuclear counterparts.
Physical Properties
Physically, heteronuclear aromatic compounds can differ significantly from homonuclear ones. The inclusion of heteroatoms generally affects the compound’s boiling point, melting point, and solubility, making them suitable for specific industrial applications that require particular physical characteristics.
Applications and Implications
In Pharmaceuticals
Aromatic compounds, especially heteronuclear ones, play a critical role in the pharmaceutical industry. Their complex structures are fundamental in the design of active pharmaceutical ingredients (APIs). These compounds provide a backbone for a wide range of drugs, including antibiotics, antiseptics, and various other therapeutic agents. Their ability to easily attach to other functional groups makes them versatile precursors in drug synthesis, allowing chemists to design molecules with specific, targeted medicinal effects.
In Materials Science
In the realm of materials science, aromatic compounds are indispensable. Their robust nature due to the aromatic ring makes them perfect candidates for developing advanced materials like high-performance plastics and synthetic fibers. For instance, Kevlar, whose strength and heat-resistance stem from its aromatic polyamide chains, is used in bulletproof vests and other high-strength materials. Similarly, aromatic polyesters and polycarbonates are used in everything from eyeglass lenses to electronic components due to their clarity, durability, and excellent insulating properties.
Environmental Impact
The environmental impact of aromatic compounds is significant and multi-faceted. On the one hand, many aromatic compounds are biologically active or toxic, necessitating careful handling and disposal to avoid environmental contamination. On the other hand, innovations in green chemistry are leading to the development of biodegradable aromatic compounds that promise to reduce environmental footprint without sacrificing performance.
Analysis Techniques
Spectroscopy Methods
Spectroscopy is key in identifying and analyzing aromatic compounds. Techniques like Nuclear Magnetic Resonance (NMR) and Infrared (IR) spectroscopy are particularly useful. NMR spectroscopy provides detailed information about the molecular structure, including the arrangement of atoms in an aromatic ring. IR spectroscopy, on the other hand, is excellent for identifying functional groups attached to the aromatic core.
Chromatography
Chromatography techniques, including gas chromatography (GC) and high-performance liquid chromatography (HPLC), are essential for purifying aromatic compounds and checking their purity. These methods are crucial in both research and industrial settings to ensure that the compounds meet the required standards for their intended uses.
Computational Modeling
Computational models play a crucial role in the study of aromatic compounds. They allow chemists to predict how new compounds might behave before synthesizing them in the lab. Computational tools can model reactions, predict stability, and even suggest new aromatic compounds with desired properties, significantly speeding up the development process.
Challenges and Solutions
Synthesis Difficulties
The synthesis of complex aromatic compounds, particularly those that are heteronuclear, can be challenging due to the precise conditions required for their formation. Innovations in catalysis and reaction conditions have led to more efficient synthetic routes. Additionally, the use of green chemistry principles is helping to make the synthesis of aromatic compounds more sustainable.
Stability Issues
While aromatic compounds are generally stable, their high reactivity can sometimes lead to instability in certain conditions, such as extreme temperatures or harsh chemical environments. Developing new derivatives of aromatic compounds that maintain stability while still being effective in their application remains a key challenge.
Future Perspectives
Research Trends
Current research in aromatic chemistry is heavily focused on the synthesis of novel aromatic compounds that are less harmful to the environment and more biologically active. There is also a strong trend towards the use of aromatic compounds in nanotechnology and electronic applications, where their unique properties can be leveraged in new ways.
Potential Applications
The potential applications for new aromatic compounds are vast. From advanced pharmaceuticals to next-generation electronic materials, the continued exploration of aromatic compounds will likely lead to significant breakthroughs in many fields. Future research will also explore how these compounds can be made more sustainably and with greater functionality.
Frequently Asked Questions
What Are Aromatic Compounds?
Aromatic compounds are a class of compounds notable for their stable ring-like structure, typically characterized by the presence of conjugated pi electrons. These structures contribute to their unique chemical properties, including a high degree of stability and distinct reactivity patterns.
Why Distinguish Homonuclear from Heteronuclear?
Distinguishing between homonuclear and heteronuclear aromatic compounds is crucial because it affects how these compounds are synthesized, manipulated, and applied in various chemical processes. Each type has unique attributes and uses, impacting everything from drug development to new material creation.
How Are Aromatic Compounds Identified?
Aromatic compounds are generally identified through techniques such as NMR spectroscopy, mass spectrometry, and IR spectroscopy. These methods allow scientists to analyze the molecular structure and confirm the aromatic nature by observing characteristic absorption patterns and molecular fragmentation.
What Are Common Uses of Heteronuclear Aromatic Compounds?
Heteronuclear aromatic compounds are widely used in pharmaceuticals and agrochemicals due to their diverse chemical properties. They are also pivotal in the synthesis of dyes, pigments, and advanced materials, offering versatility in chemical reactions and applications.
Conclusion
Aromatic compounds, both homonuclear and heteronuclear, play an indispensable role in modern chemistry and technology. Their distinct differences underscore the importance of precise chemical knowledge in developing new applications and enhancing existing ones. The ability to manipulate these compounds, grounded in a deep understanding of their structures, opens the door to innovations across scientific disciplines.
As we advance, the ongoing research and exploration of aromatic compounds will likely lead to groundbreaking discoveries and developments. Emphasizing the fundamental differences and applications of these compounds not only enriches our scientific knowledge but also propels forward the boundaries of technology and medicine.