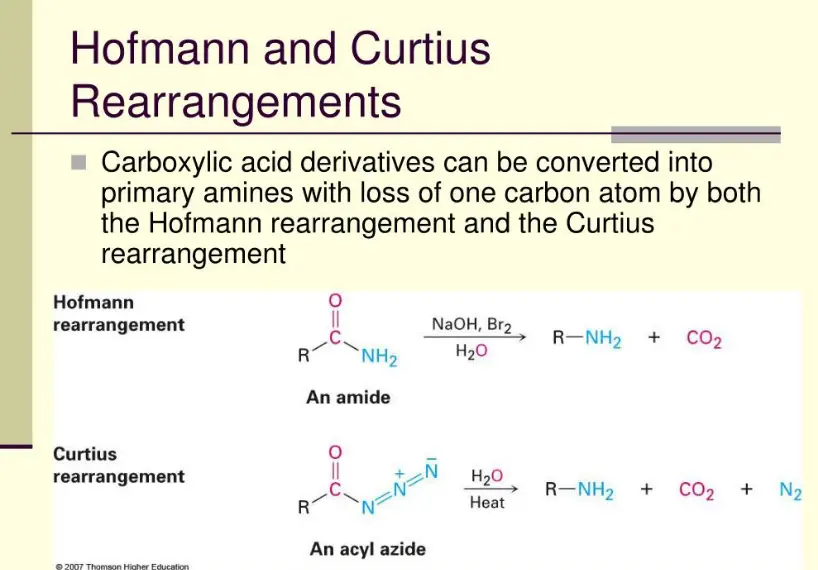
Chemical rearrangements play a pivotal role in synthetic chemistry, facilitating the transformation of molecules into more useful or reactive forms. Among the various types of rearrangements, the Hofmann and Curtius rearrangements are particularly noteworthy due to their unique mechanisms and applications. These reactions are not only fundamental in academic studies but also have practical implications in various industries.
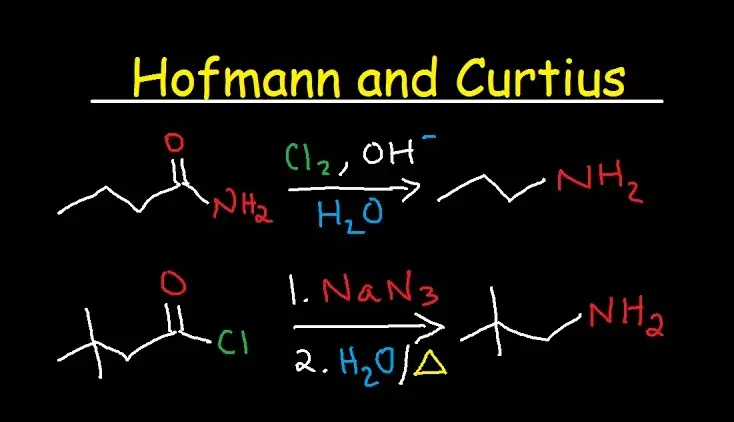
The Hofmann rearrangement involves the conversion of an amide to an amine with one fewer carbon atom, using bromine and a base. On the other hand, the Curtius rearrangement converts an acyl azide into an isocyanate, releasing nitrogen gas. Both processes are crucial for the synthesis of amines and isocyanates, which are valuable building blocks in organic chemistry.
While the Hofmann rearrangement is typically used in bulk chemical processes and medicinal chemistry, the Curtius rearrangement finds its applications in more specialized areas such as material science and the synthesis of complex natural products. Each method offers distinct advantages and challenges, making them indispensable tools in the chemist’s repertoire.
Hofmann Rearrangement
Definition and Process
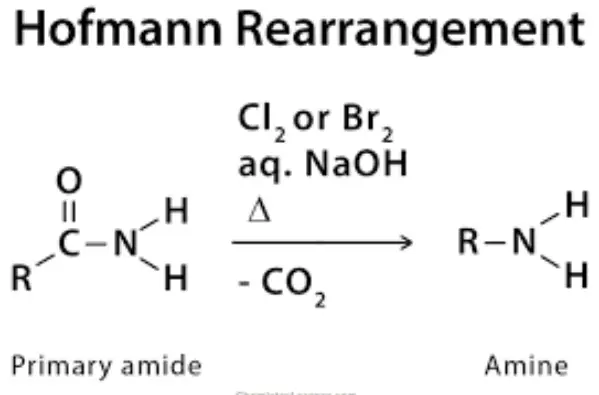
The Hofmann rearrangement is a classic organic chemistry reaction that alters the structure of an amide to produce an amine with one less carbon atom. This reaction is named after its discoverer, August Wilhelm von Hofmann, who first described it in the late 19th century. The process typically involves the use of bromine and a strong base (like sodium hydroxide) to initiate the reaction.
Chemical Reaction and Mechanism
The mechanism of the Hofmann rearrangement is fascinating due to its complexity and the precision required in its execution. Here’s how it typically unfolds:
- Step 1: The amide reacts with bromine in an aqueous solution of base, forming an intermediate N-bromoamide.
- Step 2: The N-bromoamide then undergoes deprotonation by the base, leading to the formation of a bromamide anion.
- Step 3: This anion rearranges itself, ejecting a bromide ion and forming an isocyanate intermediate.
- Step 4: The isocyanate is rapidly hydrolyzed in the presence of water, ultimately yielding the desired amine and carbon dioxide.
This reaction is not just a laboratory curiosity but a tool used to synthesize primary amines effectively.
Applications in Industry
In the industrial context, the Hofmann rearrangement has several applications, primarily due to its ability to efficiently produce amines, which are key building blocks in pharmaceuticals, agrochemicals, and dye manufacturing. For example:
- Pharmaceuticals: Many pharmaceutical compounds are derived from primary amines, making the Hofmann rearrangement valuable for synthesizing a wide range of medicinal products.
- Agrochemicals: Amines produced through this rearrangement are used to create herbicides and pesticides.
- Dye Industry: Amines are crucial in synthesizing dyes, especially in the production of azo dyes, which require primary amines.
Curtius Rearrangement
Definition and Process
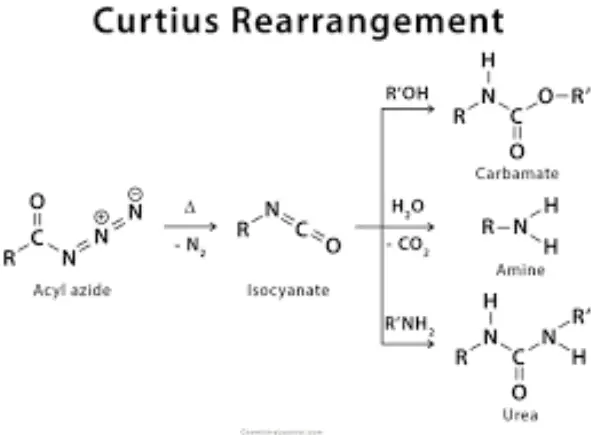
The Curtius rearrangement, named after Theodor Curtius who discovered it in 1890, involves transforming an acyl azide into an isocyanate with the release of nitrogen gas. This reaction is particularly useful for generating isocyanates without the need for harsh conditions.
Chemical Reaction and Mechanism
The Curtius rearrangement proceeds through the following steps:
- Step 1: Acyl azide is heated, causing it to decompose and form an isocyanate and nitrogen gas, a simple yet powerful transformation.
- Step 2: The resulting isocyanate can be further reacted with various nucleophiles such as water, alcohols, or amines to form ureas, carbamates, or amine products, respectively.
This reaction’s utility lies in its simplicity and the valuable compounds it helps synthesize.
Applications in Research
The applications of the Curtius rearrangement in research are broad and impactful, particularly in the fields of:
- Material Science: Isocyanates are used to manufacture polyurethane foams, elastomers, and coatings.
- Pharmaceuticals: The ability to synthesize complex molecules from simpler ones is crucial in drug development, particularly in designing compounds with specific biological activities.
- Synthetic Organic Chemistry: Researchers utilize this rearrangement to build complex molecular architectures that are otherwise challenging to synthesize.
Key Differences
Reaction Conditions
The Hofmann rearrangement typically requires aqueous or alcoholic solutions of bromine and a strong base. In contrast, the Curtius rearrangement often only needs heat to initiate the decomposition of acyl azides, making it applicable under milder conditions.
Byproducts Comparison
The Hofmann reaction generally produces carbon dioxide and bromide as byproducts, which can be advantageous or problematic, depending on the synthesis context. The Curtius reaction, however, releases only nitrogen gas—a clean byproduct which poses fewer purification challenges.
Utility in Synthesis
Both reactions are invaluable for their ability to introduce nitrogen functionalities into organic molecules, critical for synthesizing a wide range of chemical products. However, the Hofmann rearrangement is particularly useful in large-scale industrial applications due to its straightforward approach to amine synthesis. Conversely, the Curtius rearrangement shines in academic and research settings, where its versatility in forming isocyanates can be leveraged to explore new chemical territories and synthesize novel compounds.
Practical Examples
Hofmann in Medicinal Chemistry
The Hofmann rearrangement has a long-standing tradition of contributing significantly to the field of medicinal chemistry. This reaction’s ability to transform amides into amines has made it a tool of choice in the synthesis of various pharmaceutical agents. Here are some practical examples that highlight its use:
- Synthesis of Antidepressants: Many antidepressants involve primary amines in their structure. The Hofmann rearrangement is employed to synthesize these amines, which are then incorporated into drug molecules that help regulate neurotransmitter levels in the brain.
- Development of Anti-inflammatory Drugs: Nonsteroidal anti-inflammatory drugs (NSAIDs) sometimes use amines derived from the Hofmann rearrangement. These amines are integral in crafting the molecular structure that interacts effectively with biological targets to reduce inflammation.
- Production of Local Anesthetics: Primary amines, obtained via the Hofmann rearrangement, are key ingredients in several local anesthetics. These compounds temporarily block nerve conduction near their site of administration, thereby causing a numbing effect without affecting consciousness.
These applications underscore the rearrangement’s versatility and essential role in creating compounds that improve health and alleviate various medical conditions.
Curtius in Material Science
In material science, the Curtius rearrangement facilitates the development of advanced materials with unique properties. Its ability to produce isocyanates serves as a cornerstone for creating polymers and other materials critical to various industries:
- Polyurethane Foams: The isocyanates produced via the Curtius rearrangement are used to manufacture polyurethane foams. These foams are prevalent in automotive, building, and furniture applications due to their excellent insulation properties and flexibility.
- Thermoplastic Elastomers: Isocyanates are also pivotal in producing thermoplastic elastomers, which combine the performance of rubber with the processability of plastics. These materials are used in automotive parts, seals, and footwear.
- Protective Coatings: Another significant application of isocyanates from the Curtius rearrangement is in the formulation of protective coatings. These coatings are used to enhance the durability and weather resistance of outdoor structures and vehicles.
These examples illustrate the profound impact of the Curtius rearrangement in creating innovative materials that drive technological advancements.
Challenges and Solutions
Common Issues in Hofmann
Despite its widespread use, the Hofmann rearrangement is not without challenges. Some of the common issues encountered include:
- Side Reactions: The presence of excess bromine can lead to over-bromination of the substrate, thereby complicating the reaction mixture and affecting yield.
- Sensitive Substrates: Some amides are sensitive to the conditions used in the Hofmann rearrangement, leading to degradation or unwanted reactions.
- Product Purification: The formation of byproducts such as bromide and carbon dioxide can make the purification process cumbersome and less efficient.
Overcoming Curtius Rearrangement Hurdles
The Curtius rearrangement, while versatile, also presents several challenges that can affect its efficiency and applicability:
- Handling of Acyl Azides: Acyl azides, used as precursors in the Curtius rearrangement, are explosive and require careful handling and storage.
- Control of Reaction Conditions: The temperature and reaction environment need to be meticulously controlled to prevent premature decomposition of the acyl azide and ensure optimal yield of the isocyanate.
- Product Isolation: Isocyanates are highly reactive and can be challenging to isolate and purify without undergoing further unwanted reactions.
To address these challenges, researchers and chemists employ various strategies:
- Optimized Reaction Conditions: By fine-tuning the reaction conditions, such as the choice of solvent and temperature control, chemists can minimize side reactions and improve yields.
- Advanced Purification Techniques: Techniques such as distillation under reduced pressure or the use of trapping agents for isocyanates can enhance the purity of the final product.
- Use of Stabilizers: The addition of stabilizers can prevent the premature decomposition of sensitive intermediates, thereby improving the overall efficiency of the reactions.
FAQs
What is Hofmann Rearrangement?
The Hofmann rearrangement is a chemical process that converts amides into amines with the loss of one carbon atom. It uses bromine and an aqueous or non-aqueous base, facilitating a mechanism that involves the formation of an isocyanate intermediate, which is then hydrolyzed to the final amine.
How does Curtius Rearrangement work?
In the Curtius rearrangement, acyl azides are heated to form isocyanates through the expulsion of nitrogen gas. This reaction is particularly useful for generating isocyanates from carboxylic acids via an azide intermediate, which can then be utilized to synthesize ureas, carbamates, or amines through further reaction with appropriate nucleophiles.
What are the applications of these rearrangements?
Both rearrangements are primarily used to synthesize amines and other nitrogen-containing compounds. The Hofmann rearrangement is often employed in the large-scale production of pharmaceuticals and agrochemicals, whereas the Curtius rearrangement is pivotal in preparing advanced materials and designing complex molecule structures in academic research settings.
Are there any common challenges with these reactions?
One of the main challenges with the Hofmann rearrangement is the control of byproducts, particularly when dealing with sensitive substrates. The Curtius rearrangement, while versatile, requires careful handling of potentially explosive azides and the management of high-energy intermediates.
Conclusion
The Hofmann and Curtius rearrangements are cornerstone reactions in the field of organic chemistry, each with its unique set of mechanisms and applications. Their ability to efficiently transform simple precursors into more complex and functionally diverse compounds underscores their importance in both industrial and academic settings.
As the demand for more sophisticated chemical synthesis grows, the evolution of these rearrangements will continue to play a critical role in developing new technologies and materials. Understanding and optimizing these reactions are essential for future advancements in chemical manufacturing and product development.