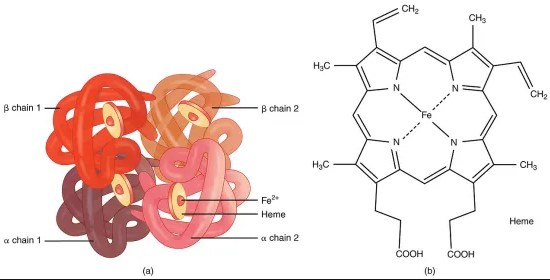
Oxygen transport is a critical process for the survival of virtually all living organisms. It involves complex proteins capable of binding and releasing oxygen molecules as they navigate through the body. Among these, hemocyanin and hemoglobin stand out not only for their essential roles but also for the fascinating differences that set them apart. These proteins, while serving the same vital function of oxygen transport, do so in remarkably distinct ways, catering to the needs of a diverse array of organisms.
Hemocyanin and hemoglobin are both oxygen-carrying proteins, but they differ significantly in their structure, the metal ions they contain, and the organisms that use them. Hemocyanin, which contains copper, is found mainly in some arthropods and mollusks, turning blue when oxygenated. Hemoglobin, on the other hand, contains iron and is prevalent in vertebrates, including humans, turning red when oxygenated. This fundamental distinction underscores the diversity of life’s strategies for sustaining itself.
The presence of these proteins in various organisms is not a random occurrence but a result of millions of years of evolution, adapting to their environments to optimize oxygen transport. Understanding the differences between hemocyanin and hemoglobin offers insights into evolutionary biology, physiology, and even potential medical applications, reflecting the ingenuity of nature in sustaining life across the planet’s vast and varied ecosystems.

Basic Definitions
Hemocyanin
What is Hemocyanin?
Hemocyanin is a copper-containing protein that functions as an oxygen carrier in the blood of certain invertebrates, notably some arthropods and mollusks. Unlike hemoglobin, which is iron-based and found in vertebrates, hemocyanin is notable for its blue color when oxygenated. It operates in a fundamentally different manner from hemoglobin, relying on copper to bind oxygen.
Organisms with Hemocyanin
Hemocyanin is primarily found in:
- Mollusks, such as octopuses, squids, and some snails.
- Arthropods, including certain species of spiders and crustaceans, like lobsters and crabs.
These organisms rely on hemocyanin for oxygen transport through their circulatory system, adapting to their specific environmental needs.
Hemoglobin
What is Hemoglobin?
Hemoglobin is an iron-containing protein that transports oxygen in the blood of vertebrates. It gives red blood cells their characteristic color and is essential for transporting oxygen from the lungs to the rest of the body and facilitating the return of carbon dioxide from tissues to the lungs.
Organisms with Hemoglobin
Hemoglobin is found in:
- Humans and other mammals
- Birds
- Fish
- Amphibians
- Reptiles
Virtually all vertebrates utilize hemoglobin for oxygen transport, making it a critical component of their circulatory system.
Molecular Structure
Hemocyanin Structure
The molecular structure of hemocyanin is fascinating and complex:
- Hemocyanin is composed of large protein units that can form extensive multimers, significantly larger than hemoglobin molecules.
- The oxygen-binding sites contain two copper ions, which directly bind to oxygen molecules.
Copper ions role
- Copper ions in hemocyanin alternate between two oxidation states (Cu(I) and Cu(II)) during the oxygenation and deoxygenation process.
- The presence of copper is what imparts the blue color to the blood of organisms with hemocyanin when oxygenated.
Hemoglobin Structure
Hemoglobin’s structure is key to its function:
- It consists of four polypeptide chains, each with an iron-containing heme group at its center.
- These heme groups are where oxygen molecules bind.
Iron ions role
- The iron ions in hemoglobin also switch between different oxidation states but primarily facilitate the binding and release of oxygen.
- This iron-based mechanism is what causes hemoglobin to turn red when it is oxygenated.
Oxygen Binding Mechanism
Hemocyanin Mechanism
The process by which hemocyanin binds oxygen is unique:
- Oxygen molecules directly bind to the copper ions at the active site of hemocyanin.
- This binding leads to a change in the protein’s structure, which can be observed as a change in color from colorless to blue.
How Hemocyanin binds with oxygen
- When oxygen is present, it binds to the copper ions, oxidizing them and causing the molecule to adopt a blue coloration.
- The removal of oxygen reverses this process, and the hemocyanin returns to its colorless state.
Hemoglobin Mechanism
Hemoglobin’s oxygen-binding mechanism is well-studied:
- Oxygen binds to the iron ions in the heme groups, causing a slight change in the hemoglobin structure.
- This change facilitates the release of oxygen to tissues that need it.
How Hemoglobin binds with oxygen
- The oxygenation of hemoglobin causes it to go from a dark red or purple to a bright red color, indicating the presence of oxygen.
- Deoxygenation reverses this process, signaling the release of oxygen to the body’s tissues.
Color Changes
Hemocyanin Color
The color change in hemocyanin is a direct indicator of its oxygenation state:
- Oxygenated hemocyanin is blue, a result of the copper ions binding to oxygen.
- Deoxygenated hemocyanin is colorless, indicating the absence of oxygen.
This color change is not just a fascinating biochemical feature but also crucial for the physiological functioning of organisms that rely on hemocyanin.
Hemoglobin Color
The color of hemoglobin is one of its most recognizable features:
- Oxygenated hemoglobin appears red, thanks to the oxygen-bound iron ions in the heme groups.
- Deoxygenated hemoglobin takes on a darker hue, often described as dark red or purple, reflecting its ready state to pick up oxygen in the lungs.
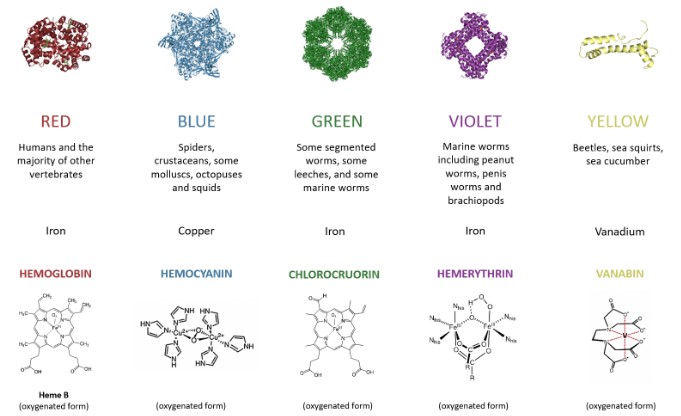
Efficiency in Oxygen Transport
Comparative Efficiency of Hemocyanin and Hemoglobin
When comparing hemocyanin and hemoglobin, it’s essential to consider their oxygen-carrying capacities and how effectively they release oxygen to tissues. Hemoglobin is highly efficient in vertebrates, supporting high metabolic rates by rapidly transporting oxygen. It’s finely tuned to the needs of warm-blooded organisms, which require constant and efficient oxygen delivery to maintain their metabolism.
Hemocyanin, on the other hand, is more suited to the slower metabolic rates of many invertebrates. While generally less efficient than hemoglobin in terms of oxygen transport capacity, hemocyanin excels in low-oxygen environments or where temperature variations are significant. Its efficiency is not just about capacity but adaptation to specific environmental contexts.
Environmental Factors Affecting Efficiency
The efficiency of both proteins is profoundly influenced by environmental factors:
- Temperature: Hemocyanin functions well in colder environments, where it can more effectively bind and release oxygen. Hemoglobin’s efficiency, while generally high, can decrease in colder temperatures.
- Oxygen availability: In areas of low oxygen concentration, hemocyanin’s affinity for oxygen allows it to still function adequately, providing a significant advantage to the organisms that rely on it.
Evolutionary Perspective
Evolution of Hemocyanin
Hemocyanin‘s evolution is a testament to nature’s adaptability. It is believed to have originated in the common ancestor of mollusks and arthropods, with its presence in these groups today reflecting a successful adaptation to their specific environmental niches. The evolution of hemocyanin showcases how life diversifies to exploit different ecological opportunities.
Evolution of Hemoglobin
Hemoglobin has a separate evolutionary path, emerging early in the vertebrate lineage. Its evolution reflects the increasing complexity of vertebrates and their need for efficient oxygen transport mechanisms to support active lifestyles and complex body structures. Hemoglobin’s versatility and adaptability have made it a cornerstone of vertebrate physiology.
Adaptive Advantages of Each
Both proteins offer adaptive advantages:
- Hemocyanin allows invertebrates to thrive in environments where oxygen levels might fluctuate significantly.
- Hemoglobin supports the high metabolic demands of vertebrates, enabling a range of lifestyles from the slow-moving to the highly active.
Applications and Research
Hemocyanin in Medicine
In recent years, hemocyanin has found novel applications in medicine, particularly in immune response and vaccine development. Its unique properties can boost the immune system’s response to various pathogens, making it a valuable tool in creating more effective vaccines. Research into hemocyanin’s applications is ongoing, with potential impacts on treating cancer and other diseases.
Hemoglobin in Medicine
Hemoglobin has long been a focus in medical research due to its central role in human physiology. Its applications include:
- Therapeutic uses: Hemoglobin-based oxygen carriers are being developed as blood substitutes in transfusions.
- Artificial blood: Research aims to create a safe, effective artificial blood product, leveraging hemoglobin’s oxygen-carrying capacity.
Both areas are of significant interest for their potential to save lives and improve medical treatments.
Environmental Adaptations
Role in Survival in Varied Habitats
The presence of hemocyanin and hemoglobin in different organisms underscores their roles in adapting to varied habitats. These adaptations are crucial for survival, enabling organisms to exploit specific ecological niches:
- Hemocyanin is often found in species that inhabit extreme or fluctuating environments, where its oxygen-binding properties offer a survival advantage.
- Hemoglobin is essential for vertebrates, supporting a wide range of activities and lifestyles, from deep-sea fish to high-flying birds.
Temperature and Pressure Effects
The effects of temperature and pressure on these proteins are significant:
- Hemocyanin‘s functionality at low temperatures and in oxygen-poor environments enables species like deep-sea squids to thrive.
- Hemoglobin‘s efficiency allows mammals and birds to maintain high metabolic rates and body temperatures, even in cold or high-altitude environments.
Frequently Asked Questions
Why do some organisms have hemocyanin instead of hemoglobin?
Some organisms have hemocyanin instead of hemoglobin due to evolutionary adaptations to their specific environments. Hemocyanin is more efficient in oxygen transport in the cold and low-oxygen conditions often found in the habitats of many mollusks and arthropods. This adaptation allows these organisms to thrive in conditions where hemoglobin-based oxygen transport might be less efficient.
Can hemocyanin be found in humans?
No, hemocyanin is not found in humans. Humans, like most vertebrates, use hemoglobin for the transport of oxygen. Hemocyanin is primarily found in certain invertebrates, including some arthropods and mollusks. The evolutionary paths of invertebrates and vertebrates diverged long ago, leading to different mechanisms for oxygen transport.
What causes the color difference between oxygenated hemocyanin and hemoglobin?
The color difference between oxygenated hemocyanin and hemoglobin is due to the different metal ions they contain and how these ions interact with oxygen. Hemocyanin contains copper, which turns blue when oxygenated, whereas hemoglobin contains iron, which turns red upon oxygenation. The specific interactions between these metal ions and oxygen molecules are responsible for the distinct colors observed.
How does the efficiency of oxygen transport compare between hemocyanin and hemoglobin?
The efficiency of oxygen transport between hemocyanin and hemoglobin can vary significantly depending on the environmental conditions. Hemoglobin is generally more efficient at oxygen transport in warm-blooded animals and under conditions with ample oxygen. However, hemocyanin can be more efficient in cold and low-oxygen environments, which is why it is found in some arthropods and mollusks that inhabit such conditions.
Conclusion
The exploration of hemocyanin and hemoglobin uncovers the complexity and diversity of life’s strategies for surviving and thriving on Earth. Through understanding the differences and similarities between these two proteins, we gain insights into the evolutionary paths that have shaped the living world. This knowledge not only enriches our understanding of biology but also opens avenues for medical and scientific advancements.
Reflecting on the broader implications, the study of oxygen-transport proteins exemplifies the beauty of nature’s design. It reveals how life adapts to its environment, evolving mechanisms that are as varied as they are efficient. In appreciating these differences, we recognize the intricate balance and interdependence that define life on our planet, underscoring the importance of preserving its diversity for future generations.