Hydrogen sulfide (H2S) and sulfur dioxide (SO2) are two chemical compounds that, despite sharing a part of their names and being sulfur-related, possess distinct characteristics and implications for health and the environment. Their differing properties mean they behave uniquely in natural and industrial settings, making it crucial to distinguish between them accurately. Both compounds are commonly encountered in various industries, including oil and gas, wastewater treatment, and manufacturing, where their management is key to operational safety and environmental protection.
The primary difference between H2S and SO2 lies in their chemical composition and the subsequent impact on human health and the environment. H2S is a colorless gas with a notorious rotten egg odor, highly toxic and flammable, mainly resulting from organic matter decomposition. On the other hand, SO2, also a colorless gas but with a sharp, burning odor, primarily comes from the burning of fossil fuels and is known for its role in acid rain formation. Understanding these differences is essential for handling these substances safely and mitigating their environmental impact.
Distinguishing H2S from SO2 is not just a matter of chemical curiosity but a necessity for ensuring the safety of workers and populations exposed to these gases and for the protection of the environment. Both gases require specific detection, handling, and treatment technologies due to their distinct properties and health implications. The conversation around H2S and SO2 is also deeply intertwined with discussions on air quality, industrial safety, and environmental legislation, reflecting their significant impact across multiple spheres.
Chemical Properties
H2S Composition
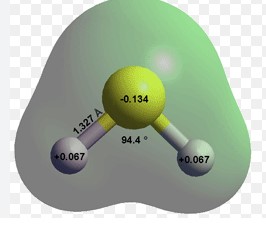
Hydrogen sulfide (H2S) is a simple molecular compound consisting of two hydrogen atoms bonded to a single sulfur atom. Its molecular structure is akin to that of water (H2O), with hydrogen atoms forming a bent shape around the sulfur, giving it a distinct angular geometry. This structure is critical in determining its chemical behavior and physical properties.
- Basic Structure: Linear with a bent shape due to the two hydrogen atoms
- Characteristics: Colorless gas, highly toxic, and carries a characteristic rotten egg smell. It is also flammable and can form explosive mixtures in air.
SO2 Composition
Sulfur dioxide (SO2), in contrast, comprises one sulfur atom double-bonded to two oxygen atoms. This arrangement results in a bent molecular shape, similar to H2S but with oxygen instead of hydrogen. The presence of oxygen atoms significantly alters the compound’s reactivity and physical properties.
- Basic Structure: Bent molecular shape with a sulfur atom centrally located
- Characteristics: Colorless gas with a sharp, choking odor. It is not flammable but is considered toxic and can be a significant pollutant in the atmosphere.
Comparison
When comparing H2S and SO2, the atomic differences lie primarily in the substitution of hydrogen in H2S for oxygen in SO2. This change significantly impacts their chemical behaviors, particularly in how they interact with other substances and their role in environmental processes.
- Atomic Differences: Hydrogen (in H2S) vs. Oxygen (in SO2)
- Similarities: Both are colorless gases and exhibit a bent molecular shape, contributing to their distinctive properties.
Physical Properties
H2S Traits
The physical state of H2S under normal conditions is a colorless gas. It is infamous for its foul odor, reminiscent of rotten eggs, making it easily identifiable at low concentrations. This characteristic smell is often the first indicator of H2S presence in an area.
- Physical State: Gas
- Color: Colorless
- Odor: Rotten eggs
SO2 Traits
SO2 is also a colorless gas at room temperature. However, its odor is markedly different from H2S, described as sharp and burning, akin to a struck match. This pungent smell can make exposure to SO2 particularly unpleasant.
- Physical State: Gas
- Color: Colorless
- Odor: Sharp, burning
Side-by-Side Analysis
A direct comparison of H2S and SO2’s physical properties reveals both similarities and distinctions important for identification and handling.
- Boiling Points: H2S has a boiling point of -60°C, making it more volatile than SO2, which boils at -10°C.
- Densities: H2S is lighter than air, whereas SO2 is heavier, influencing their dispersion in the environment.
- Solubility: SO2 is more soluble in water than H2S, leading to different behaviors in aqueous environments.
Health Effects
H2S Exposure
Exposure to H2S, even at low concentrations, can have severe health implications. Short-term exposure may result in eye irritation, coughing, and nausea, while long-term or high-level exposure can lead to more severe conditions such as lung damage, nervous system effects, and even death.
SO2 Exposure
SO2 affects health differently, primarily targeting the respiratory system. Short-term exposure can cause throat and eye irritation, coughing, and shortness of breath. Prolonged exposure increases the risk of respiratory diseases like asthma and chronic bronchitis.
Preventive Measures
To mitigate the health risks associated with H2S and SO2 exposure, specific safety protocols and equipment are recommended:
- Personal Protective Equipment (PPE): Includes gas masks and respirators for breathing protection.
- Ventilation Systems: Essential in industrial settings to dilute and remove hazardous gases.
- Monitoring and Detection: Use of sensors to detect gas presence and concentrations in real-time, allowing for prompt action.
Environmental Impact
H2S in the Environment
Hydrogen sulfide (H2S) is naturally present in the environment, originating from organic matter decomposition under anaerobic conditions such as in swamps, sewers, and volcanic gases. While it plays a role in the natural sulfur cycle, excessive amounts can have detrimental effects on ecosystems. High concentrations of H2S can lead to oxygen depletion in water bodies, affecting fish and aquatic life. In the atmosphere, H2S can oxidize to form sulfur dioxide (SO2), contributing to acid rain and air pollution.
SO2 in the Environment
Sulfur dioxide (SO2) is primarily released into the environment through the burning of fossil fuels containing sulfur compounds, industrial processes, and volcanic eruptions. It poses significant environmental risks, particularly as a major contributor to acid rain. Acid rain can lead to the acidification of lakes and streams, damaging aquatic habitats and reducing biodiversity. SO2 in the atmosphere can also harm plant life by damaging foliage and inhibiting growth.
Mitigation Strategies
Global and Local Efforts
Mitigating the environmental impacts of H2S and SO2 involves a combination of global treaties and local regulations aimed at reducing emissions. Strategies include:
- Cleaner Production Techniques: Adoption of cleaner, more efficient industrial processes to reduce sulfur emissions.
- Emission Controls: Installation of scrubbers and other technologies in power plants and factories to remove sulfur compounds from exhaust gases.
- Renewable Energy: Encouraging the use of renewable energy sources to reduce reliance on fossil fuels.
Industrial Applications
H2S Uses
Despite its toxicity, H2S has important uses in various industries:
- Petroleum Industry: H2S is removed from natural gas and used in the production of sulfuric acid, a valuable industrial chemical.
- Waste Treatment: Utilized in sewage treatment plants to minimize odor and corrosion.
SO2 Uses
SO2’s chemical properties make it valuable in several applications:
- Preservative: In the food industry, SO2 is used to preserve freshness and prevent microbial growth.
- Bleaching Agent: Used in paper and textile manufacturing to bleach materials.
- Chemical Manufacturing: Integral in the production of sulfuric acid and other chemicals.
Economic Implications
Market Dynamics and Sustainability
The industrial demand for sulfur compounds drives a significant market, influenced by environmental regulations and advances in technology. The shift towards sustainability is prompting industries to adopt cleaner technologies and alternative chemicals, potentially impacting the market dynamics of H2S and SO2.
Detection and Measurement
H2S Detection
Detecting H2S accurately is crucial for safety and environmental monitoring. Technologies include:
- Electronic Sensors: Offer real-time monitoring but may require frequent calibration.
- Colorimetric Tubes: Provide instant readings but are single-use and can be less accurate over time.
SO2 Detection
For SO2, detection methods must account for its presence in both industrial settings and the atmosphere:
- Spectroscopy: Utilized for atmospheric monitoring, measuring the absorption of light by SO2.
- Electrochemical Sensors: Common in industrial applications for their sensitivity and specificity.
Accuracy and Limitations
Challenges in Detection and Measurement
Detecting and measuring H2S and SO2 come with challenges:
- Interference: Other gases can interfere with sensor readings, reducing accuracy.
- Sensitivity: Detecting low concentrations accurately is crucial for health and environmental monitoring.
- Durability: Sensors and devices must withstand harsh environments.
Legal and Regulatory Framework
H2S Regulations
Globally, regulations on H2S focus on occupational exposure limits and emission controls. Standards and guidelines vary by country but aim to protect workers and the public from the health risks associated with H2S.
SO2 Regulations
For SO2, international agreements like the Kyoto Protocol aim to reduce atmospheric concentrations. National regulations often set limits on emissions from power plants and factories to protect air quality and public health.
Compliance Challenges
Industry Adaptation and Enforcement Issues
Ensuring compliance with H2S and SO2 regulations poses challenges:
- Cost: Implementing emission control technologies and safety measures can be expensive.
- Monitoring and Enforcement: Effective regulation requires robust monitoring and enforcement mechanisms.
- Global Consistency: Achieving consistent standards and enforcement across countries remains a challenge.
Frequently Asked Questions
What are the main sources of H2S and SO2?
The main sources of hydrogen sulfide (H2S) include the decomposition of organic matter, particularly in anaerobic environments such as swamps and sewers, and industrial processes like petroleum refining. Sulfur dioxide (SO2), conversely, primarily originates from the burning of fossil fuels containing sulfur, such as coal and oil, and from volcanic emissions. Both gases are byproducts of various industrial processes, making their management crucial in industrial settings.
How do H2S and SO2 affect human health?
Hydrogen sulfide (H2S) is highly toxic, even at low concentrations. Exposure can lead to a range of health effects, from mild irritation of the eyes and throat to serious impacts such as shock, convulsions, and, in extreme cases, death. Sulfur dioxide (SO2) primarily affects the human respiratory system, causing coughing, mucus secretion, and aggravation of conditions like asthma and bronchitis. Long-term exposure to SO2 can lead to respiratory illness and can affect the lung function.
What are the environmental impacts of H2S and SO2?
Hydrogen sulfide (H2S) can contribute to the greenhouse gas effect and, upon oxidation, can form sulfur dioxide, contributing indirectly to acid rain. Sulfur dioxide (SO2), more directly, plays a significant role in acid rain formation, which can lead to the acidification of soils and water bodies, harming plant and aquatic life. SO2 is also a precursor to particulate matter that can cause visibility impairment and contribute to atmospheric pollution.
How are H2S and SO2 detected and measured?
Detection and measurement of hydrogen sulfide (H2S) and sulfur dioxide (SO2) are commonly conducted using specialized sensors and analytical techniques. For H2S, colorimetric tubes, electrochemical sensors, and gas chromatography are frequently used. For SO2, ultraviolet fluorescence, infrared spectroscopy, and again gas chromatography serve as common methods. Accurate detection and quantification are critical for safety, regulatory compliance, and environmental monitoring.
Conclusion
Recognizing the difference between hydrogen sulfide (H2S) and sulfur dioxide (SO2) is not merely an academic exercise but a practical necessity with significant implications for public health, industrial safety, and environmental stewardship. These compounds, while chemically related, exhibit markedly different behaviors and impacts in our world, necessitating tailored approaches to their management and mitigation.
As we continue to navigate the complexities of industrial processes and their environmental footprints, understanding the nature and effects of substances like H2S and SO2 becomes increasingly important. By educating ourselves and implementing robust safety and environmental protection measures, we can mitigate the risks associated with these gases, safeguarding both human health and the natural world.