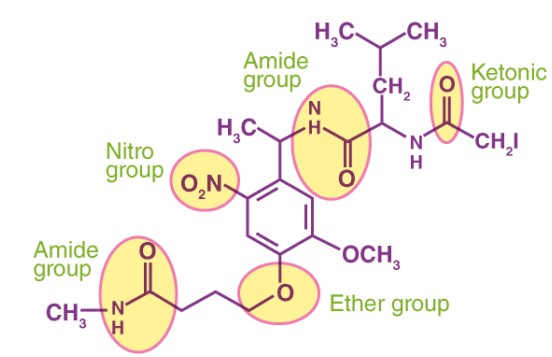
Organic chemistry, the study of carbon-containing compounds, introduces a vast array of molecular structures, each with unique properties and reactivities. At the heart of this diversity are functional groups and substituents, critical components that dictate how a molecule behaves in chemical reactions and in its physical environment. These elements serve as the alphabet of organic chemistry, allowing scientists to read and write the complex language of molecules.
The difference between a functional group and a substituent lies in their roles and effects on a molecule. A functional group is a specific cluster of atoms within a molecule that has a characteristic pattern of reactivity, dictating how the molecule participates in chemical reactions. On the other hand, a substituent is any atom or group of atoms that replaces a hydrogen atom in a hydrocarbon, primarily influencing the molecule’s physical properties rather than its chemical reactivity.
This distinction is not merely academic; it has practical implications in fields ranging from pharmaceuticals to material science. Functional groups are the active centers that undergo chemical transformations, while substituents can alter a compound’s solubility, boiling point, and overall molecular shape. Understanding the nuances between these two components is crucial for manipulating and predicting the behavior of organic molecules in various applications.
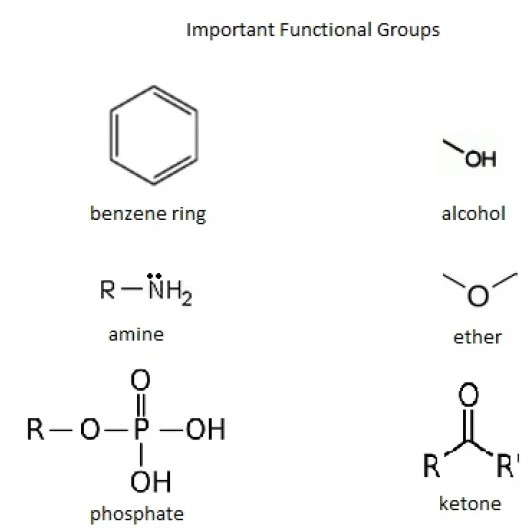
Core Concepts
What is a Functional Group?
In organic chemistry, functional groups are specific clusters of atoms within molecules that are pivotal in determining how these molecules engage in chemical reactions. These groups, characterized by a consistent set of reactivity patterns, are the backbone of molecular behavior in chemical processes. For instance, the presence of a hydroxyl group (-OH) can make a compound alcoholic, bestowing it with certain chemical behaviors distinct from those of compounds with other functional groups.
Key Characteristics
- Reactivity: Functional groups define the reaction pathways a molecule can take.
- Predictability: They allow for the prediction of molecular behavior in chemical reactions.
- Classification: Molecules are often classified based on their functional groups.
Examples
- Hydroxyl Group (-OH): Found in alcohols, making them soluble in water.
- Carbonyl Group (>C=O): Key in ketones and aldehydes, affecting their boiling points and reactivity.
- Amino Group (-NH2): Central to amino acids, influencing their ability to form proteins.
What is a Substituent?
Substituents are atoms or groups of atoms that replace hydrogen atoms on a parent hydrocarbon chain. Unlike functional groups, their primary influence is on the physical properties of molecules, such as boiling and melting points, without markedly changing the molecule’s chemical reactivity. Substituents can be as simple as a single chlorine atom or as complex as a benzene ring attached to the main chain.
Role in Molecules
- Physical Properties: Substituents affect properties like solubility and melting point.
- Molecular Structure: They can alter the shape and size of molecules, influencing how molecules interact with each other.
Examples
- Methyl Group (-CH3): A simple substituent affecting the molecule’s hydrophobicity.
- Ethyl Group (-C2H5): Another hydrocarbon substituent, altering boiling points.
- Halogen Atoms (Cl, Br, I): Can significantly change a molecule’s reactivity and physical properties.
Key Differences
Reactivity vs. Properties
The distinction between functional groups and substituents lies primarily in their impact on a molecule. Functional groups are directly involved in chemical reactions, defining the types of chemical processes a molecule can participate in. Substituents, however, play a more subtle role, often tweaking the physical properties of molecules, which can indirectly influence how those molecules react under certain conditions.
Structural Role
Functional groups create reactive sites within a molecule, serving as the active centers for chemical reactions. In contrast, substituents modify the molecule’s structure without introducing new sites for chemical reactivity. This structural modification can influence a molecule’s physical behavior and interactions but does not directly contribute to its chemical transformations.
Types and Examples
Functional Groups
- Hydroxyl Group (-OH): Makes alcohols polar and soluble in water.
- Carboxyl Group (-COOH): Gives carboxylic acids their acidic properties.
- Amine Group (-NH2): Found in amines, affecting their basicity.
Substituents
- Methyl Group (-CH3): Increases hydrophobicity.
- Chloro Group (-Cl): Adds density and affects boiling point.
- Phenyl Group (C6H5-): Aromatic ring that influences reactivity and physical properties.
Influence on Molecular Behavior
The presence of either a functional group or a substituent can significantly alter the behavior of a molecule, but through different pathways. Functional groups are the main actors in chemical reactions, dictating the ways in which a molecule can react and interact with other substances. Substituents, on the other hand, adjust the stage on which these reactions occur, by altering physical properties like solubility, which can affect how molecules come together in a reaction mixture.
Molecular behavior is thus a concert between the chemical reactivity introduced by functional groups and the physical context provided by substituents. Understanding both is crucial for mastering the intricacies of organic chemistry and for the effective design and manipulation of molecular structures for specific purposes.
Comparative Analysis
Chemical Reactions
The role of functional groups and substituents in dictating chemical reactions is pivotal, shaping the course and outcome of these reactions. Functional groups act as the reactive centers of molecules, engaging directly in chemical processes that lead to the formation of new compounds. For example, the carboxylic acid group (����COOH) can undergo reactions such as esterification, where reacting with an alcohol in the presence of an acid catalyst produces an ester. This reaction is characteristic of the carboxylic acid functional group, regardless of the molecule it’s part of.
In contrast, substituents modify the reactivity of the molecule by their electronic effects (inductive or resonance effects) and steric hindrance, without being the direct participants in the reaction. A methyl group (��3CH3), for example, might increase the electron density of an adjacent functional group through inductive effect, subtly altering its reactivity. However, the methyl group itself does not react; it influences the reactivity of the functional group it is attached to.
Molecular Structure
The representation of molecular structures in organic chemistry emphasizes the importance of both functional groups and substituents. Functional groups are often depicted as the distinctive parts of the molecule, with their specific connectivity and orientation highlighted. For instance, the amine group (��2NH2) is shown with its nitrogen atom bonded to one or more carbon atoms, indicating its potential for forming hydrogen bonds and engaging in nucleophilic substitution reactions.
Substituents, on the other hand, are shown as variations from the parent hydrocarbon structure. They are depicted based on their position and the type of atom or group replacing a hydrogen atom. The bromine substituent (��Br), for example, is indicated by its attachment point on the carbon chain, affecting the molecule’s physical properties like polarity and boiling point but not fundamentally altering the molecule’s reactivity pattern.
Impact on Physical Properties
Substituents and functional groups distinctly influence the physical properties of molecules. Substituents primarily affect properties like boiling point, melting point, and solubility by altering the molecular structure’s size, shape, and polarity. A fluoro substituent (�F), for instance, can significantly increase a molecule’s polarity, thereby increasing its solubility in polar solvents.
Functional groups, due to their reactive nature, can have a more profound impact on a molecule’s reactivity. However, their presence can also influence physical properties through mechanisms like hydrogen bonding. An alcohol group (��OH), for example, can engage in hydrogen bonding, substantially affecting the molecule’s boiling point and solubility in water.
Real-World Applications
Pharmaceutical Design
In pharmaceutical design, the distinction between functional groups and substituents becomes crucial. The presence of specific functional groups in a drug molecule can confer the desired biological activity, dictating how the drug interacts with biological targets. For instance, the hydroxyl group is often involved in forming hydrogen bonds with amino acid residues in enzymes or receptors, a critical interaction for the drug’s efficacy.
Substituents, while not directly involved in the drug’s interaction with its biological target, can modify the drug’s pharmacokinetic properties, such as absorption, distribution, metabolism, and excretion (ADME). Adjusting substituents can enhance a drug’s solubility or stability, making it more effective or easier to administer.
Material Science
In material science, the knowledge of functional groups and substituents enables the design of materials with specific properties. Polymers, for example, can be synthesized with particular functional groups to impart desired characteristics like flexibility, strength, or chemical resistance. Substituents can be used to adjust the polymer’s properties further, such as its thermal stability or solubility in different solvents.
Environmental Chemistry
Understanding functional groups and substituents is also vital in environmental chemistry, where it helps predict the environmental fate of chemicals. Functional groups can determine a compound’s reactivity with environmental agents like water, oxygen, or sunlight, leading to degradation pathways that minimize environmental impact. Substituents can influence the bioaccumulation potential and toxicity of compounds, guiding the design of safer chemicals.
Frequently Asked Questions
What is a functional group in chemistry?
A functional group in chemistry is a specific grouping of atoms within a molecule that behaves in a predictable manner, typically undergoing similar chemical reactions regardless of the molecule it is part of. These groups are responsible for the characteristic properties and reactivity patterns of organic compounds, making them key to understanding and designing chemical reactions.
How do substituents affect a molecule?
Substituents affect a molecule by altering its physical properties, such as boiling point, melting point, and solubility, without significantly changing the molecule’s core reactivity. They can influence the molecule’s shape, size, and how it interacts with other molecules, thereby affecting its behavior in a given environment or under specific conditions.
Can a functional group act as a substituent?
Yes, a functional group can act as a substituent when it is attached to the main chain of a larger molecule, thereby modifying the properties of the larger molecule. In this context, the functional group’s characteristic reactivity can influence the overall reactivity of the molecule, in addition to impacting its physical properties.
Why is understanding the difference between functional groups and substituents important?
Understanding the difference between functional groups and substituents is crucial for predicting and manipulating the chemical behavior of organic compounds. This knowledge allows chemists to design molecules with desired properties and reactivities, facilitating advancements in pharmaceuticals, material science, and environmental chemistry, among other fields.
Conclusion
Grasping the distinction between functional groups and substituents unlocks the potential to predict and influence the behavior of organic molecules, a foundational aspect of chemistry that resonates through various scientific disciplines. This understanding not only propels research and innovation but also enhances our ability to create compounds tailored for specific purposes, from medicine to materials.
In conclusion, while functional groups and substituents may seem like minor details in the vast world of organic chemistry, their impact on a molecule’s reactivity and properties is profound. As we continue to explore the intricacies of molecular behavior, these elements remain central to our ability to harness the power of chemistry for solving complex challenges and advancing human knowledge.