Nuclear isotopes play a pivotal role in the fields of energy production and scientific research, with particular focus on their capabilities within nuclear reactors. These isotopes, categorized mainly into fissile and fertile, hold unique properties that dictate their utility in nuclear fission processes. Their characteristics determine how they can be used to sustain or initiate nuclear chain reactions, essential for both energy generation and medical isotopes production.
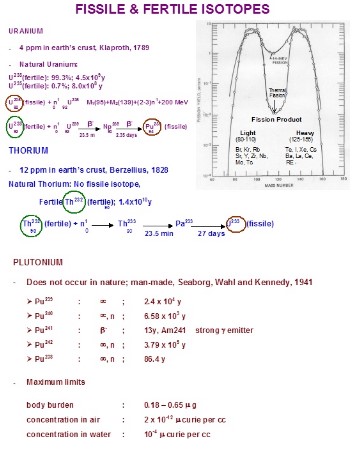
Fissile isotopes can sustain chain reactions with neutrons of any energy, the most notable being Uranium-235 and Plutonium-239. In contrast, fertile isotopes, such as Uranium-238 and Thorium-232, cannot initiate a fission reaction on their own but can absorb neutrons to transform into fissile isotopes. This conversion is crucial for the sustainability of nuclear energy, providing a pathway to generate more fuel from otherwise non-fissionable isotopes.
The distinction between fissile and fertile isotopes is not just academic but has practical implications for how nuclear reactors are designed and operated. Fissile isotopes are primarily used to sustain energy-producing reactions, whereas fertile isotopes are valuable for their potential to breed new fissile material, offering a resource-efficient method to extend the life of nuclear fuel.
Fissile Isotopes
Definition
Fissile isotopes are types of nuclear isotopes capable of sustaining a nuclear chain reaction when struck by free neutrons. The ability to sustain a chain reaction distinguishes fissile materials from other types of nuclear materials, making them critical for nuclear power generation.
Characteristics
Spontaneous Fission
One remarkable characteristic of fissile isotopes is their ability to undergo spontaneous fission. This process involves the nucleus of an atom splitting into two smaller nuclei, releasing neutrons and a large amount of energy. Spontaneous fission is relatively rare compared to neutron-induced fission but is crucial for understanding the stability and safety of nuclear materials.
Sustaining Chain Reactions
Fissile materials are primarily sought after for their ability to sustain chain reactions. This property is essential for the operation of nuclear reactors, where controlled chain reactions are used to produce energy. The neutrons released during the fission process can initiate further fissions, leading to a continuous release of energy.
Common Examples
Uranium-235
Uranium-235 is perhaps the most well-known fissile isotope, widely used in nuclear reactors and atomic bombs. It has a relatively high probability of fission upon absorbing a slow-moving neutron, making it highly effective for energy production.
Plutonium-239
Plutonium-239 is another key fissile material, created from Uranium-238 through neutron capture and subsequent beta decay. It is extremely potent and requires only a small amount to sustain a chain reaction, playing a crucial role in both power generation and nuclear weapons.
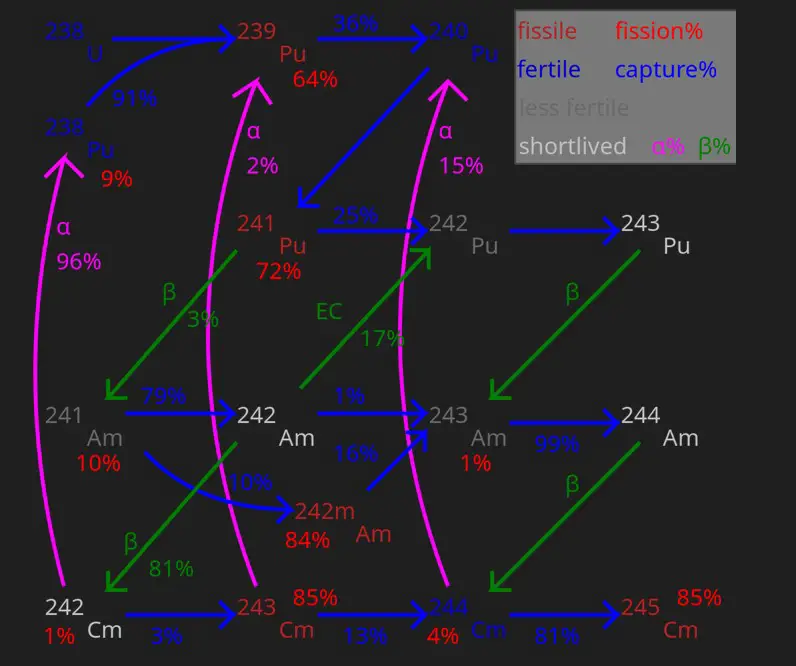
Fertile Isotopes
Definition
Fertile isotopes are those nuclear materials that, while not capable of sustaining a nuclear chain reaction themselves, can be transformed into fissile materials through neutron absorption and subsequent nuclear reactions.
Characteristics
Neutron Absorption
Fertile isotopes absorb neutrons to initiate a transformation process. This absorption is the first step in converting a fertile isotope into a fissile one, essential for breeding new nuclear fuel from spent or depleted resources.
Conversion Process
The conversion of fertile isotopes into fissile isotopes typically involves several stages of neutron capture followed by beta decay. This transformation is fundamental to the operation of breeder reactors, which aim to produce more fissile material than they consume.
Common Examples
Uranium-238
Uranium-238, the most abundant isotope of uranium found in nature, is a prime example of a fertile isotope. While it cannot sustain a chain reaction, it can be converted into Plutonium-239, a highly fissile material.
Thorium-232
Thorium-232, another significant fertile isotope, undergoes a series of reactions to become Uranium-233, a fissile isotope. This process is key to the Thorium fuel cycle, which is being explored as a safer and more abundant alternative to the Uranium fuel cycle.
Comparison Overview
Key Differences
Reaction Types
The fundamental difference between fissile and fertile isotopes lies in their reaction types. Fissile isotopes can undergo fission just from the absorption of a neutron, while fertile isotopes require conversion into fissile isotopes before they can participate in fission reactions.
Role in Reactors
Fissile isotopes are direct contributors to the energy output in nuclear reactors, essential for maintaining a sustained and controlled chain reaction. On the other hand, fertile isotopes serve as a resource for breeding new fissile material, enhancing the efficiency and longevity of fuel use in reactors.
Conversion Processes
From Fertile to Fissile
The conversion of fertile isotopes to fissile materials involves neutron absorption and a series of nuclear reactions, each contributing to the transformation. This process not only sustains the chain reaction necessary for ongoing energy production but also maximizes the utilization of available nuclear materials, reducing waste and increasing fuel sustainability.
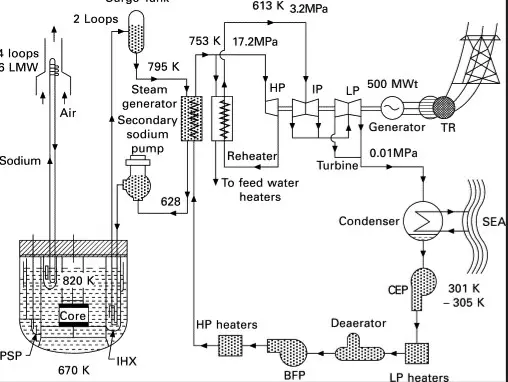
Applications in Energy
Nuclear Reactors
Types Using Fissile Materials
Nuclear reactors harness the power of fissile materials like Uranium-235 and Plutonium-239 to generate energy. These reactors function by maintaining a controlled chain reaction where neutrons split the nucleus of these isotopes, releasing heat and more neutrons to sustain the process. There are several types of reactors based on the use of fissile isotopes:
- Pressurized Water Reactors (PWRs): Use water under high pressure as a coolant to keep the reactor core at a lower temperature.
- Boiling Water Reactors (BWRs): Boil water with the heat from nuclear fission, which directly drives the steam turbines.
- Fast Neutron Reactors (FNRs): Use fast neutrons to sustain fission, with a higher efficiency in fuel usage compared to thermal reactors.
Breeder Reactors
Breeder reactors are a special type of nuclear reactor that utilizes fertile isotopes, such as Uranium-238 and Thorium-232, to produce more fissile material than they consume. These reactors play a crucial role in ensuring the sustainability of nuclear energy by generating additional fissile material:
- Fast Breeder Reactors (FBRs): Operate with fast neutrons and have a core surrounded by a fertile material blanket where new fissile atoms are bred.
- Thermal Breeder Reactors: Less common, these reactors use thermal neutrons to convert fertile isotopes into fissile material.
Safety and Regulation
Safety Measures
Nuclear safety is paramount, encompassing rigorous measures that ensure the operation and management of nuclear materials are conducted without significant risk to operators, the public, and the environment. Key safety measures include:
- Controlled Access: Restricting access to nuclear materials to qualified personnel only.
- Routine Inspections: Frequent checks to ensure all systems and components meet safety standards.
- Emergency Protocols: Well-defined procedures in case of an accident to mitigate risks and manage nuclear fallout.
Handling and Transportation
Handling and transportation of nuclear materials are performed under strict regulations to prevent accidents and unauthorized access. These materials are transported in specially designed containers that shield radiation and prevent the release of nuclear material in case of transport accidents.
International Regulations
Globally, nuclear activities are governed by several treaties and organizations such as the International Atomic Energy Agency (IAEA) which sets international safety standards and assists countries in nuclear safety and security.
- Monitoring and Control: International oversight on nuclear materials and technology to prevent proliferation and ensure safe usage.
Environmental Impact
Waste Management
Managing nuclear waste is a critical environmental challenge. Nuclear reactors produce radioactive waste that must be handled with care:
- Temporary Storage: Waste is initially cooled in spent fuel pools at reactor sites.
- Permanent Disposal: High-level waste is destined for deep geological repositories where it can be isolated for thousands of years.
Disposal Methods
- Reprocessing: Some waste can be reprocessed to extract usable materials, reducing the volume of final waste.
- Encapsulation: Waste is encapsulated in glass or ceramic within secure casks for storage or disposal.
Long-term Effects
- Radiation: Potential leaks from waste disposal sites can lead to environmental contamination.
- Contamination: Persistent contamination can affect ecosystems and human health for generations.
Future Prospects
Research and Development
Ongoing research focuses on improving reactor efficiency, safety, and waste management. Innovations like small modular reactors (SMRs) and generation IV reactors promise enhanced safety and less waste.
Innovations in Isotope Use
The development of new reactor technologies and isotopic applications in medicine and industry continue to advance, broadening the scope of nuclear technology beyond energy production.
Global Energy Contributions
Nuclear energy remains a key component of the global energy mix, offering a stable and low-carbon energy source. Future trends suggest a balanced approach, integrating nuclear power with renewable energy sources to meet the world’s energy demands sustainably.
Frequently Asked Questions
What is a fissile isotope?
A fissile isotope is capable of sustaining a nuclear chain reaction with neutrons of any energy level. Common fissile isotopes include Uranium-235 and Plutonium-239, both critical to the operation of most nuclear reactors.
What makes an isotope fertile?
An isotope is considered fertile when it can absorb neutrons and undergo nuclear reactions to transform into a fissile material. Key examples include Uranium-238 and Thorium-232, which are converted into fissile isotopes through neutron absorption and subsequent nuclear transformations.
How do fissile and fertile isotopes differ?
The primary difference between fissile and fertile isotopes lies in their ability to sustain chain reactions. Fissile isotopes can directly sustain chain reactions, while fertile isotopes must first transform into fissile isotopes through neutron absorption and decay processes.
Why are fertile isotopes important in nuclear reactors?
Fertile isotopes are crucial for breeding reactors as they can be converted into fissile isotopes, thus producing more fuel from the same amount of material. This process significantly enhances the efficiency and sustainability of nuclear energy resources.
Conclusion
Understanding the differences between fissile and fertile isotopes provides essential insight into the operational dynamics of nuclear reactors and the long-term sustainability of nuclear energy. These isotopes not only dictate the current landscape of nuclear power but also influence future advancements in reactor technology.
The ongoing research and development in this field aim to optimize the use of these isotopes, enhancing safety measures, and minimizing environmental impact. As the world leans more towards sustainable energy solutions, the role of both fissile and fertile isotopes will undoubtedly become more crucial, underscoring the importance of continued innovation in nuclear technology.