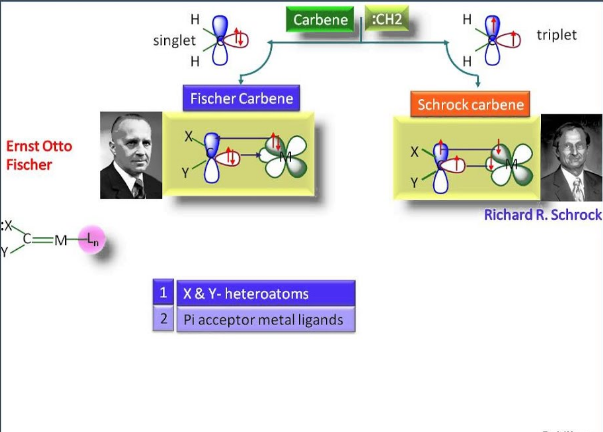
Carbenes, highly reactive species in organic chemistry, play pivotal roles in numerous synthetic transformations. These neutral molecules feature a divalent carbon atom with two non-bonded electrons, making them extremely versatile in their reactivity. Among the various types of carbenes, Fischer and Schrock carbenes stand out due to their distinct structures and applications.
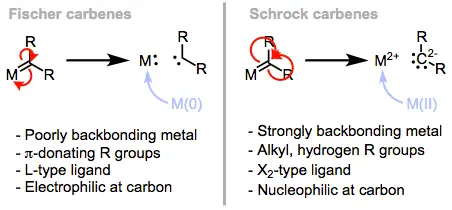
Fischer carbenes are typically characterized by an electron-rich carbene carbon bonded to an electron-withdrawing group and a metal, usually a transition metal like chromium. On the other hand, Schrock carbenes generally involve a high-valent metal such as molybdenum or tungsten and feature a carbene carbon bonded to electron-donating groups. These structural differences significantly impact their stability and reactivity, making them suitable for different chemical reactions.
Both Fischer and Schrock carbenes are foundational in the development of complex molecules and have broad applications ranging from pharmaceuticals to material science. Their unique reactivities enable chemists to construct and manipulate molecules in innovative ways that are otherwise challenging or inefficient with other reagents.
Carbene Basics
Definition and Characteristics
Carbenes are unique, reactive intermediates in organic chemistry, characterized by a neutral carbon atom with two non-bonded electrons. This configuration leads to significant electron deficiency, making carbenes highly reactive and short-lived in their natural state. Typically, the carbon in a carbene is sp2 hybridized, allowing it to form a linear or nearly linear geometry with adjacent atoms, contributing to its distinctive reactivity.
Common Uses in Chemistry
Carbenes are instrumental in a variety of chemical reactions, primarily due to their ability to insert into carbon-hydrogen (C-H) and other types of bonds. Key applications include:
- Olefin Metathesis: Facilitates the rearrangement of carbon-carbon double bonds.
- C-H Activation: Allows the modification of hydrocarbons, which is crucial in pharmaceutical synthesis.
- Cyclopropanation: Used in the synthesis of cyclopropane rings, valuable in medicinal chemistry.
Fischer Carbene
Composition and Structure
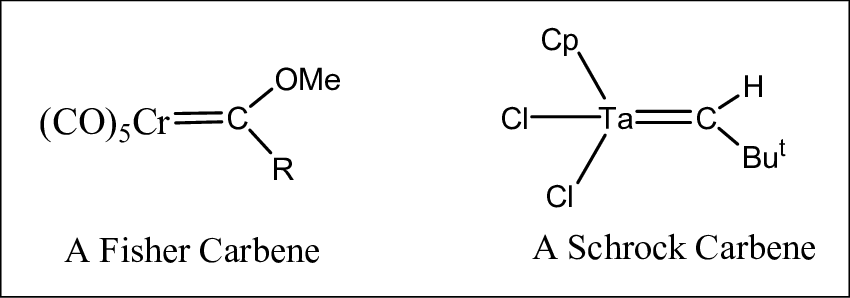
Fischer carbenes are a class of carbene complexes where the carbene carbon is bonded to a transition metal and an electron-withdrawing group (EWG), such as an alkoxy or amido group. These carbenes are typically depicted with a double bond between the metal and the carbene carbon, though this representation is more symbolic and reflects the carbene’s resonance structures rather than a true double bond.
Typical Reactions and Applications
Fischer carbenes are versatile in organic synthesis. Their main reactions include:
- Addition to Carbonyl Groups: Fischer carbenes can add to aldehydes and ketones, forming new carbon-carbon bonds.
- Insertion into C-H Bonds: They are used to modify alkanes, enhancing molecular complexity.
These reactions are beneficial in synthesizing complex molecular architectures often used in drug development and materials science.
Stability Factors
The stability of Fischer carbenes is greatly influenced by the nature of the attached metal and the electron-withdrawing group. The presence of a strong EWG helps stabilize the carbene by delocalizing its lone pair of electrons through resonance, reducing its reactivity and extending its usable lifespan in reactions.
Schrock Carbene
Composition and Structure
Schrock carbenes, named after Richard R. Schrock, a Nobel laureate, typically consist of a high-valent metal such as tungsten, molybdenum, or tantalum bonded to a carbene carbon that is adjacent to electron-donating groups (EDGs). Unlike Fischer carbenes, Schrock carbenes do not involve a metal-carbon double bond and are often involved in more aggressive reactions due to their higher reactivity.
Typical Reactions and Applications
Schrock carbenes are primarily known for their role in:
- Olefin Metathesis Reactions: Essential for polymer and petrochemical industries.
- Alkene Formation: Useful in constructing large, complex organic structures.
These reactions are crucial for synthesizing polymers and chemicals in an efficient and environmentally friendly manner.
Stability Factors
The stability of Schrock carbenes is less than that of Fischer carbenes, largely due to the presence of electron-donating groups that make the carbene carbon more electron-rich and reactive. Their reactivity, however, can be controlled by the choice of metal and its oxidation state, as well as the steric and electronic properties of the ligands involved.
Key Differences
Electronic Properties
The electronic differences between Fischer and Schrock carbenes are primarily influenced by their ligands. Fischer carbenes are electron-poor due to their EWGs, while Schrock carbenes are electron-rich, owing to their EDGs. This fundamental disparity affects how each type of carbene interacts with other molecules.
Reactivity Comparisons
Fischer carbenes are generally more stable and less reactive than Schrock carbenes, making them suitable for more controlled organic syntheses. Schrock carbenes, with their higher reactivity, are better suited for rapid and robust transformations, such as in industrial applications.
Stability and Lifespan
Stability in carbene chemistry is pivotal for their practical use. Fischer carbenes, with their stable configurations, are more suited for reactions requiring precision, while the transient nature of Schrock carbenes makes them ideal for fast reactions in dynamic conditions. This difference in stability and lifespan is crucial for chemists when selecting the appropriate carbene for a specific synthesis or application.
Applications in Chemistry
Role in Organic Synthesis
Carbenes, particularly Fischer and Schrock carbenes, play a critical role in organic synthesis, where their unique reactivity enables the formation of complex molecular structures. Fischer carbenes are particularly useful for:
- Alkene Additions: They facilitate the addition across double bonds to form new, functionalized organic compounds.
- Cycloadditions: Useful in creating ring structures, which are foundational in many natural products and pharmaceuticals.
Schrock carbenes, on the other hand, excel in:
- Ring-Opening Metathesis Polymerization (ROMP): Ideal for creating polymers with specific properties.
- Cross Metathesis: Enables the formation of disubstituted alkenes by reacting two different alkenes.
These applications highlight the versatility of carbenes in synthesizing a wide array of chemical products, from small molecules to large polymers.
Use in Industrial Processes
In industrial settings, the unique characteristics of carbenes allow for their use in a variety of processes:
- Material Science: Schrock carbenes are used in the production of advanced materials, including specialty plastics and performance polymers.
- Pharmaceutical Manufacturing: Fischer carbenes are employed in the synthesis of complex drug molecules, often involving chiral intermediates that are challenging to produce through other means.
Comparative Analysis
Advantages of Each Type
Fischer and Schrock carbenes each offer distinct advantages:
- Fischer Carbenes: Offer greater control and precision in reactions due to their stability. This makes them ideal for delicate synthesis processes where exact outcomes are crucial.
- Schrock Carbenes: Their high reactivity is beneficial for rapid and efficient synthesis, particularly in bulk chemical processes where speed is a priority.
Limitations and Challenges
Despite their benefits, both types of carbenes face certain limitations:
- Fischer Carbenes: Their need for strong electron-withdrawing groups can limit the scope of their use, particularly in reactions where such groups interfere with the desired outcome.
- Schrock Carbenes: The high reactivity can also be a drawback, as it may lead to unwanted side reactions or require stringent conditions to control the reactivity.
Future Perspectives
Recent Advancements
Recent advancements in carbene chemistry have expanded their utility and efficiency. Innovations include:
- Stabilized Carbenes: Development of more stable carbene forms that can be stored and used under less stringent conditions.
- Catalytic Applications: Increased use of carbenes in catalysis, including their role in catalytic converters in automotive technology.
These developments have not only enhanced the fundamental understanding of carbene chemistry but also their practical applications in various industries.
Potential Developments in Carbene Research
Looking ahead, the potential developments in carbene research are promising:
- Greener Chemistry: Efforts to use carbenes in more environmentally friendly processes are gaining traction, focusing on minimizing toxic byproducts and reducing energy consumption.
- Biomedical Applications: Research is ongoing into the use of carbenes for biomedical applications, including drug delivery mechanisms and diagnostic agents.
Frequently Asked Questions
What are Carbenes?
Carbenes are reactive intermediates with a neutral carbon atom that has two non-bonded electrons. Their high reactivity makes them valuable in various chemical reactions, particularly in the formation of new bonds.
How do Fischer Carbenes differ from Schrock Carbenes?
Fischer Carbenes are typically electron-rich and are stabilized by an electron-withdrawing group and a metal. Schrock Carbenes, conversely, are stabilized by an electron-donating group and are associated with a high-valent metal, affecting their reactivity and applications.
What are the uses of Fischer and Schrock Carbenes?
Fischer and Schrock carbenes are extensively used in organic synthesis. Fischer carbenes are often utilized in the formation of heterocycles, while Schrock carbenes are pivotal in metathesis reactions, crucial for forming polymers.
Why is stability important in carbene chemistry?
Stability in carbene chemistry is crucial because it determines the lifespan and reactivity of the carbene, which in turn influences the types of reactions the carbene can participate in and its effectiveness as a reagent in organic synthesis.
Conclusion
The distinction between Fischer and Schrock carbenes encapsulates a fascinating aspect of modern chemistry, highlighting the versatility and complexity of carbene compounds. Their differences in electronic properties, stability, and reactivity not only influence their practical applications but also underscore the nuanced approaches chemists must take when implementing these reagents in synthesis.
Understanding these carbenes’ unique characteristics allows chemists to more effectively harness their properties for the development of innovative materials and pharmaceuticals. As research continues to evolve, the breadth of applications and the efficiency of these carbenes are expected to expand, further solidifying their importance in the chemical sciences.