Halogenation reactions stand as pivotal processes in organic chemistry, transforming simple organic compounds into more complex, functionalized molecules. Among these, the Finkelstein and Swarts reactions are particularly notable for their utility in synthesizing alkyl halides. These reactions not only exemplify the versatility of halogenation but also showcase the nuanced differences that can significantly affect synthetic strategies.
The Finkelstein reaction specifically replaces an alkyl chloride or bromide with an alkyl iodide, using sodium iodide in acetone. Conversely, the Swarts reaction is renowned for introducing fluorine in place of other halogens, utilizing various fluorinating agents. This distinction underscores their unique roles in the realm of organic synthesis, highlighting the specific conditions under which each reaction proceeds and their respective utilities.
These reactions embody the intricate balance of reactivity and selectivity, serving as fundamental tools for chemists to modify organic molecules. By understanding their mechanisms, conditions, and applications, one gains insight into not only these processes but also the broader field of halogenation reactions. This understanding is crucial for advancing the synthesis of complex molecules in pharmaceuticals, materials science, and beyond.
Finkelstein Reaction
Basics
Definition
The Finkelstein Reaction is a halogen exchange process in organic chemistry that converts alkyl chlorides or bromides into alkyl iodides. This transformation is facilitated by the reaction of the alkyl halide with sodium iodide in a polar aprotic solvent, typically acetone. The reaction is driven by the precipitation of sodium chloride or sodium bromide, which are insoluble in the reaction medium.
Reaction Mechanism
The mechanism of the Finkelstein reaction involves a nucleophilic substitution (SN2 mechanism):
- The iodide ion from sodium iodide acts as a nucleophile, attacking the carbon atom bonded to the leaving group (chloride or bromide).
- This leads to the displacement of the leaving group and the formation of a new carbon-iodine bond, resulting in the alkyl iodide product.
Conditions
Solvents Used
- Acetone is the most commonly used solvent for this reaction because of its ability to dissolve sodium iodide while precipitating sodium chloride or bromide.
- Other polar aprotic solvents like acetonitrile can also be used depending on the solubility of the reactants.
Temperature Requirements
- The reaction is typically carried out at room temperature.
- Temperature can be adjusted to speed up the reaction or to manage the solubility of the reactants and products.
Applications
Synthesis Examples
- Synthesis of alkyl iodides: Transforming less reactive alkyl chlorides or bromides into more reactive alkyl iodides, which are valuable intermediates in organic synthesis.
- Labeling compounds with iodine: Introducing iodine into molecules for analytical or medicinal purposes.
Utility in Organic Chemistry
- The Finkelstein reaction is highly valued for its simplicity, efficiency, and selectivity in introducing iodine into organic molecules.
- It serves as a fundamental tool in the synthesis of various organic compounds, including pharmaceuticals and agrochemicals.
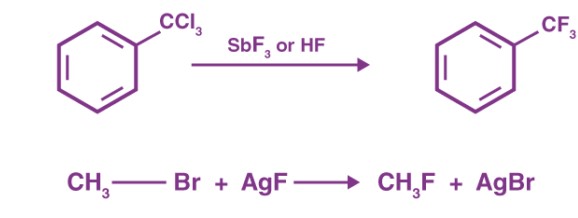
Swarts Reaction
Fundamentals
Definition
The Swarts Reaction is a fluorination process that replaces a halogen atom in an organic molecule with a fluorine atom. This reaction employs fluorinating agents like antimony trifluoride (SbF3), silver fluoride (AgF), or sulfur tetrafluoride (SF4) to achieve the substitution.
Mechanistic Insight
The mechanism involves a nucleophilic substitution similar to the Finkelstein reaction but tailored for the introduction of fluorine:
- The fluorinating agent provides a fluoride ion, which acts as a nucleophile.
- The fluoride ion attacks the carbon bonded to the original halogen (chlorine, bromine, or iodine), displacing it and forming a new carbon-fluorine bond.
Required Conditions
Fluorinating Agents
- Antimony trifluoride (SbF3): Commonly used for its reactivity and ease of handling.
- Silver fluoride (AgF) and sulfur tetrafluoride (SF4): Also used based on the specific requirements of the reaction.
Temperature and Solvents
- Temperature can vary but is often conducted at elevated temperatures to ensure the reactivity of the fluorinating agents.
- Solvents are chosen based on their ability to dissolve the starting material and the fluorinating agent, with common choices including chloroform and dichloromethane.
Uses
Industrial Applications
- Fluorination of pharmaceuticals: Enhancing the bioavailability and stability of drugs.
- Production of agrochemicals: Creating more effective pesticides and herbicides.
Role in Fluorochemicals Production
- Manufacturing of fluoropolymers: Essential for creating materials with high chemical and thermal resistance.
- Synthesis of refrigerants: Key in the development of more efficient and environmentally friendly cooling systems.
Key Differences
Reactivity
- The Finkelstein reaction specifically targets the substitution of chloride or bromide ions with iodide, while the Swarts reaction focuses on replacing various halogens with fluorine.
Halides Involved
- Iodide is the halogen introduced in the Finkelstein reaction.
- Fluorine is the halogen introduced in the Swarts reaction.
Conditions and Solvents
- The Finkelstein reaction is notable for its use of acetone as a solvent, facilitating the precipitation of by-products.
- The Swarts reaction requires solvents that can dissolve the starting material and fluorinating agents, with a preference for non-polar solvents.
Applications
- Divergent uses in chemistry: While the Finkelstein reaction is widely used for synthesizing alkyl iodides, essential in organic synthesis and labeling, the Swarts reaction is critical in the production of fluorinated compounds, pivotal in pharmaceuticals, agrochemicals, and materials science.
Practical Aspects
Laboratory Considerations
Safety Measures
When conducting Finkelstein and Swarts reactions, safety is paramount due to the use of reactive halogenated compounds and potentially hazardous conditions:
- Use of gloves and goggles is mandatory to prevent skin and eye contact with harmful chemicals.
- Fume hoods should always be used to avoid inhalation of volatile organic compounds and toxic gases.
- Proper disposal of halogenated waste is crucial to prevent environmental contamination.
Scale-up Potential
Scaling up these reactions from a laboratory to industrial scale involves significant considerations:
- Reaction monitoring to ensure consistency and safety at larger volumes.
- Efficient separation and purification techniques to handle increased quantities of products and by-products.
- Automation and control systems for maintaining optimal reaction conditions.
Real-World Applications
Pharmaceuticals
Both reactions have profound implications in the pharmaceutical industry:
- Finkelstein reaction is used to synthesize iodine-containing drugs that are crucial for thyroid health and imaging.
- Swarts reaction facilitates the production of fluorinated pharmaceuticals, which often have enhanced efficacy and stability.
Material Science
In material science, these reactions contribute to the development of advanced materials:
- Fluoropolymers, with exceptional chemical and thermal resistance, are manufactured using principles from the Swarts reaction.
- Organic light-emitting diodes (OLEDs) and other electronic materials benefit from the precise halogenation offered by these reactions.
Comparative Analysis
Efficiency and Yield
- The Finkelstein reaction is renowned for its high yield of alkyl iodides, thanks to the insolubility of sodium chloride/bromide in acetone.
- The Swarts reaction, while efficient for introducing fluorine, often requires careful control of conditions to achieve high purity products.
Reaction Rates
- Reaction rates for Finkelstein can be very fast, depending on the reactants and solvent used.
- Swarts reactions may proceed at a slower rate, influenced by the fluorinating agent and the substrate’s reactivity.
Product Purity
- Product purity in Finkelstein reactions is generally high due to the straightforward separation of products from the precipitated salts.
- In Swarts reactions, achieving high purity might require additional purification steps, especially when using more reactive fluorinating agents.
Environmental Impact
Waste Production
- Both reactions generate halogenated waste, which must be handled and disposed of with care to minimize environmental impact.
- Efforts are underway to develop greener alternatives or modifications to these reactions to reduce waste.
Green Chemistry Aspects
- Recycling of solvents and reagents is explored to make these reactions more sustainable.
- Research focuses on finding less hazardous fluorinating agents for the Swarts reaction and solvent-free conditions for the Finkelstein reaction.
Advancements and Innovations
Recent Studies
Recent studies have introduced innovations in both reactions:
- Catalysts have been developed to increase the efficiency and selectivity of the Finkelstein reaction.
- Eco-friendly fluorinating agents have been explored in the Swarts reaction to reduce the environmental footprint.
Modifications for Enhanced Reactivity
- Microwave irradiation has been used to enhance the rate of the Finkelstein reaction.
- Ionic liquids as solvents for the Swarts reaction offer improved reactivity and easier purification processes.
Future Directions
Potential Areas of Research
The ongoing research in these fields aims to address the challenges and limitations of the Finkelstein and Swarts reactions:
- Developing non-toxic and biodegradable fluorinating agents for the Swarts reaction.
- Enhancing the scalability and environmental friendliness of the Finkelstein reaction for industrial applications.
- Investigating new applications in synthesis, such as in the production of organic semiconductors and biomolecules.
FAQs
What is the Finkelstein reaction?
The Finkelstein reaction is a halogen exchange process in organic chemistry that converts alkyl chlorides or bromides into alkyl iodides. This is achieved by treating the starting material with sodium iodide, typically in a solvent like acetone. The reaction’s success hinges on the solubility of sodium chloride or bromide in acetone, which drives the equilibrium toward the formation of the desired alkyl iodide.
How does the Swarts reaction differ from Finkelstein?
The Swarts reaction is primarily used for the fluorination of organic compounds, replacing a heavier halogen (like chlorine or bromine) with fluorine. This reaction utilizes fluorinating agents such as antimony trifluoride or silver fluoride. Unlike the Finkelstein reaction, which is specific to the introduction of iodine, the Swarts reaction focuses on integrating fluorine into organic molecules, showcasing a different aspect of halogenation chemistry.
What are the applications of these reactions in industrial chemistry?
Both the Finkelstein and Swarts reactions find extensive applications in industrial chemistry, particularly in the synthesis of agrochemicals, pharmaceuticals, and materials. The Finkelstein reaction is crucial for producing iodine-containing compounds, often used as key intermediates in organic synthesis. The Swarts reaction, on the other hand, is vital for manufacturing fluorinated organic compounds, which are important in several sectors, including the pharmaceutical industry for their bioactive properties and in materials science for creating high-performance polymers.
Conclusion
The Finkelstein and Swarts reactions represent essential methodologies within organic chemistry, each serving distinct purposes in the synthesis and modification of organic compounds. Their differences highlight the diversity of halogenation reactions and their critical roles in advancing the field of synthetic chemistry. Through the careful application of these reactions, chemists can selectively introduce halogens into organic molecules, facilitating the development of novel compounds with a wide range of applications.
Understanding these reactions and their nuances not only enriches our knowledge of chemical processes but also opens up avenues for innovation in various industries. As the field of chemistry continues to evolve, the strategic application of such reactions will remain indispensable, underlining the importance of mastering these fundamental techniques for future advancements in science and technology.

