Chemical bonds form the backbone of molecular structure, pivotal to the behaviors and properties of compounds. Among these, double bonds stand out due to their role in defining the reactivity and stability of molecules. This article focuses on the differences between two types of double bonds: exocyclic and endocyclic, which are crucial for understanding organic chemistry’s complex landscapes.
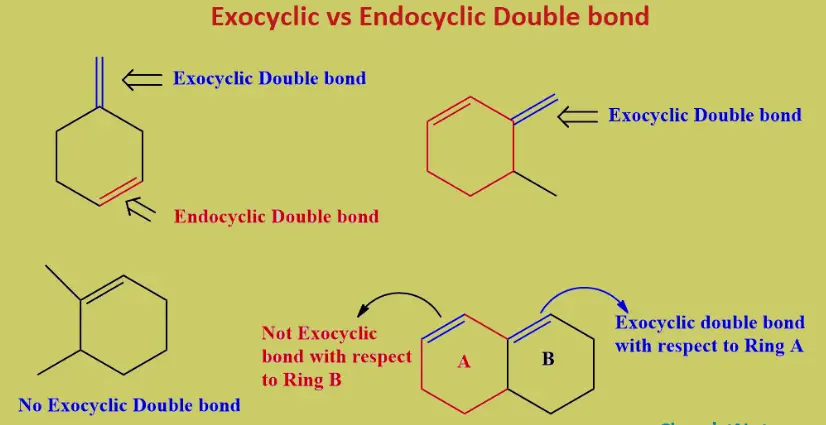
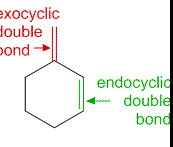
Exocyclic and endocyclic double bonds differ primarily in their placement within a molecule. An exocyclic double bond occurs when at least one of the bonded atoms is part of a ring structure but extends outside the ring. Conversely, an endocyclic double bond is completely enclosed within a ring structure. These placements significantly influence the chemical characteristics and applications of the molecules they are part of.
In organic chemistry, the position of a double bond within cyclic compounds can alter a molecule’s reactivity, dictate its synthesis pathways, and impact its functionality in biological systems. Recognizing these differences is essential for chemists who design pharmaceuticals, develop new materials, and study biological systems.
Double Bonds Basics
What is a Double Bond?
A double bond in chemistry is a type of chemical bond where two pairs of electrons are shared between two atoms. Unlike a single bond, which involves just one electron pair, double bonds are characterized by the sharing of two electron pairs, giving them distinctive properties and reactivities. This bond formation is common between carbon atoms in organic compounds but can also occur between various other elements.
Key Characteristics
Double bonds are stronger and shorter than single bonds, making them significant in molecular structure. The presence of a double bond can significantly affect a molecule’s shape, the angles between atoms, and the molecule’s overall stability. Additionally, double bonds introduce rigidity into a molecule; the atoms involved in the double bond cannot rotate freely around the bond axis, which has implications in the 3D shape of molecules.
- Reactivity: Double bonds are generally more reactive than single bonds due to the presence of the second electron pair which can be attacked by electrophiles or participate in various chemical reactions.
- Electron Density: High electron density makes double bonds prone to attack by electrophiles.
- Polarity: Depending on the attached groups, double bonds can be polar, influencing how a molecule interacts with other substances.
Role in Molecular Structure
Double bonds play a crucial role in defining the architecture of chemical compounds. In cyclic compounds, for instance, the presence of a double bond can stabilize the ring structure by reducing angle strain. Moreover, the rigidity introduced by double bonds is essential in biological molecules like DNA, where the double bond in the base pairs maintains the helix structure’s integrity and significantly influences the molecule’s properties and functions.
Exocyclic Double Bonds
Definition and Explanation
An exocyclic double bond occurs when at least one member of the double-bonded atoms extends outside a ring structure. This arrangement contrasts with endocyclic double bonds, which are entirely contained within a ring. Exocyclic bonds can influence the physical and chemical properties of a compound significantly.
Common Examples
- Styrene: A classic example where the double bond between the vinyl group and the benzene ring is exocyclic.
- Cholesterol: Contains an exocyclic double bond in one of its side chains, crucial for its biological function.
Impact on Molecular Behavior
Exocyclic double bonds affect molecular behavior in several ways:
- Reactivity: These bonds often exhibit enhanced reactivity because they are more accessible to reactants than their endocyclic counterparts.
- Conformation: The presence of an exocyclic double bond can restrict the rotation around other bonds, affecting the overall shape and reactivity of the molecule.
Endocyclic Double Bonds
Definition and Explanation
An endocyclic double bond is located entirely within a cyclic structure. This positioning can influence the stability and chemical properties of the ring, often making it less reactive than exocyclic double bonds.
Typical Occurrences
- Benzene: Perhaps the most well-known example with three endocyclic double bonds that contribute to its exceptional stability and aromaticity.
- Cyclohexene: Features a single endocyclic double bond, impacting its chemical reactivity and physical properties.
Influence on Chemical Properties
Endocyclic double bonds contribute to:
- Stability: The endocyclic placement often leads to more stable structures due to the uniform distribution of electrons across the ring.
- Ring Strain: Less strain is typically observed in rings with endocyclic double bonds compared to those with exocyclic double bonds, influencing how these molecules behave under various conditions.
Comparative Analysis
Structural Differences
The structural variance between exocyclic and endocyclic double bonds significantly impacts the design and function of organic molecules. Exocyclic double bonds extend outside of a ring structure, adding complexity to the molecule’s shape and influencing the way it interacts with other molecules. In contrast, endocyclic double bonds are contained within ring structures, often contributing to a more rigid and planar molecule.
- Exocyclic Bonds: Lead to more flexible molecular structures that can adopt multiple conformations.
- Endocyclic Bonds: Contribute to a fixed, often planar structure that restricts flexibility but enhances stability.
Chemical Properties Contrast
The chemical properties of molecules vary depending on whether a double bond is exocyclic or endocyclic:
- Polarity and Solubility: Exocyclic double bonds might alter the polarity of a molecule more significantly, affecting its solubility in different solvents.
- Stability: Endocyclic double bonds typically provide greater thermal stability to a molecule compared to exocyclic double bonds due to the reduced strain within the ring.
Reactivity Comparison
The placement of the double bond affects a molecule’s reactivity:
- Electrophilic Addition Reactions: Exocyclic double bonds are often more reactive towards electrophiles because they are more exposed and less sterically hindered.
- Participation in Ring-Opening Reactions: Endocyclic double bonds are more likely to participate in reactions that lead to ring opening, which is crucial in many organic synthesis processes.
Implications in Organic Synthesis
Role in Drug Design
Understanding the nuances of exocyclic and endocyclic double bonds is critical in drug design. The position of a double bond within a drug molecule can influence its biological activity, how it interacts with the target in the body, and its metabolic stability.
- Exocyclic Bonds: Often used to modify the reactivity and decrease the metabolic degradation of drug molecules.
- Endocyclic Bonds: Can increase molecular stability, making the drug more resistant to metabolic breakdown.
Importance in Material Science
In material science, the role of double bonds, particularly in polymers and small organic molecules, dictates the mechanical properties and durability of materials.
- Thermoset Plastics: Often involve endocyclic double bonds that, once formed, create a rigid structure ideal for materials requiring high thermal stability.
- Elastomers: Typically utilize exocyclic double bonds, allowing for greater flexibility and elasticity in materials like rubber.
Examples in Natural Products
Natural products, such as vitamins and hormones, often contain exocyclic or endocyclic double bonds which are key to their function and reactivity.
- Vitamin A: Contains an exocyclic double bond crucial for its role in vision and cell growth.
- Steroids: Feature endocyclic double bonds that are vital for their biological activities.
Visual Examples
Diagrams of Exocyclic and Endocyclic Bonds
Visual representations help illustrate the differences between exocyclic and endocyclic double bonds:
- Structural Models: Showing
the atomic arrangement in molecules like styrene (with an exocyclic double bond) and cyclohexene (with an endocyclic double bond) highlights how the bonds affect the overall shape and electron distribution within the molecule.
Case Studies Highlighting Differences
Analyzing specific case studies can elucidate how the placement of double bonds influences chemical behavior and application:
- Case Study on Prostaglandins: These are a group of physiologically active lipid compounds having diverse hormone-like effects in animals. Prostaglandins can include both exocyclic and endocyclic double bonds, affecting their interaction with biological receptors and their synthesis pathways.
- Synthesis of Aromatic Compounds: Many aromatic compounds, such as pharmaceutical intermediates, involve transitions from exocyclic to endocyclic double bonds in their synthesis processes, showcasing different reactivity and mechanisms.
Frequently Asked Questions
What is an Exocyclic Double Bond?
An exocyclic double bond occurs when one or more of the atoms involved in the double bond is connected to a ring system but extends beyond the ring itself. This configuration can affect the molecule’s physical properties and its reactivity in chemical reactions.
How Does an Endocyclic Double Bond Differ?
Endocyclic double bonds are located entirely within a ring structure of a molecule. This positioning often stabilizes the molecule but may limit its reactivity compared to exocyclic double bonds due to the constrained ring system.
Why are Double Bond Locations Important?
The location of a double bond within a molecule determines its chemical properties and reactivity. It influences how a molecule interacts with other compounds, its stability, and its role in larger chemical reactions, making it a critical consideration in synthetic chemistry and drug design.
Can Double Bonds Affect Molecular Stability?
Yes, the presence and position of double bonds significantly impact molecular stability. Endocyclic double bonds can enhance stability due to ring strain relief, whereas exocyclic double bonds may introduce reactive sites that are more prone to chemical interactions.
Conclusion
The distinction between exocyclic and endocyclic double bonds provides a foundational understanding of how molecular structures can vary dramatically in organic chemistry. The placement of these bonds affects everything from a molecule’s stability to its reactivity, influencing practical applications in various scientific fields.
Recognizing the differences between these bond types not only aids in the synthesis of new compounds but also enhances the predictability of chemical behavior in complex reactions. Such knowledge is crucial for advancing pharmaceuticals, creating innovative materials, and understanding biological mechanisms at a molecular level.

