Phase changes in matter are fascinating processes that showcase the dynamic nature of substances around us. Among these transformations, the transition from liquid to gas holds particular intrigue due to its vital role in both natural phenomena and human-made systems. This transition can occur through two primary processes: evaporation and vaporization. While closely related, these processes have distinct characteristics and implications that are crucial to understand.
Evaporation is a surface phenomenon where molecules from a liquid escape into the gas phase at a temperature below the boiling point of the liquid. Vaporization, on the other hand, refers to the change from liquid to gas that happens at the boiling point, encompassing both boiling and sublimation. These processes are driven by different conditions and have varying energy requirements, impacting their rates and the environments in which they occur.
Understanding the differences between evaporation and vaporization enhances our grasp of various natural and technological processes. From the water cycle that sustains life on Earth to the engineering of cooling systems and the distillation of spirits, these phase changes are integral to the environment and industry. By examining the conditions under which each process occurs and their implications, we gain insights into their significance and applications.

Basics of Phase Change
What Is Phase Change?
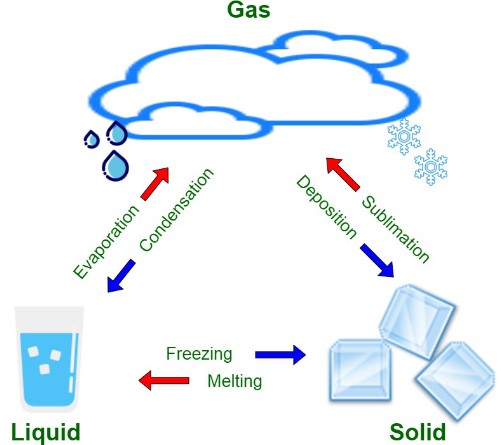
A phase change is a transformation that material undergoes when it changes from one state of matter to another. These states—solid, liquid, and gas—represent different phases of matter. The transition between these states can occur in various ways, depending on the conditions such as temperature and pressure. A common example of a phase change is water turning into ice or steam.
Factors Influencing Phase Change
Several factors can influence the occurrence and nature of a phase change:
- Temperature: The most direct factor affecting phase changes. Heating or cooling a substance changes its energy levels and can cause it to transition between solid, liquid, and gas.
- Pressure: Altering the pressure on a substance can lead to phase changes. For example, increasing the pressure on ice causes it to melt at temperatures below 0°C (32°F).
- Impurities: The presence of impurities in a substance can lower or raise its freezing and boiling points, affecting the conditions under which phase changes occur.
Understanding Evaporation
Definition of Evaporation
Evaporation is the process through which liquid turns into gas at temperatures below its boiling point. This occurs when molecules at the surface of a liquid gain enough energy to break free from their liquid bonds and escape into the air as gas.
Characteristics of Evaporation
- Surface Phenomenon: Evaporation happens at the surface of a liquid.
- Temperature Below Boiling Point: It occurs without the liquid reaching its boiling point.
- Cooling Effect: As the more energetic molecules leave the liquid, the average kinetic energy decreases, causing a cooling effect.
- Spontaneous: Evaporation can happen naturally, without any external energy source.
Common Examples
- Drying of Wet Clothes: Water from wet clothes evaporates into the air, leaving the fabric dry.
- Sweating and Cooling: Humans and animals use evaporation for cooling. Sweat evaporates from the skin, absorbing heat and cooling the body.
- Evaporation of Puddles: After rain, puddles gradually disappear as water evaporates into the atmosphere.
Exploring Vaporization
Definition of Vaporization
Vaporization is a general term for the phase change from liquid to gas. This can occur through boiling or sublimation, depending on the conditions.
Types of Vaporization
Boiling
Boiling is a form of vaporization where a liquid turns to gas at its boiling point and occurs throughout the liquid, not just on the surface.
Characteristics of Boiling
- Occurs at Boiling Point: The temperature at which a liquid boils and turns into gas.
- Bubbles: Gas bubbles form throughout the liquid and rise to the surface.
- Energy Intensive: Requires significant energy input to reach the boiling point.
Sublimation
Sublimation is the transition of a substance from solid to gas without passing through the liquid state.
Characteristics of Sublimation
- Bypasses Liquid State: Directly goes from solid to gas.
- Occurs Under Specific Conditions: Typically requires low pressure or specific temperatures.
- Used in Various Processes: Such as freeze-drying food and making dry ice.
Key Differences
Temperature Conditions
- Evaporation can happen at any temperature below the boiling point of the liquid.
- Vaporization (boiling) requires the liquid to reach its boiling point.
Energy Requirements
- Evaporation requires less energy compared to boiling, as only surface molecules need enough energy to escape.
- Vaporization requires substantial energy to bring the entire volume of the liquid to its boiling point.
Rate of Process
- Evaporation is generally a slower process as it depends on the surface area and ambient conditions.
- Boiling is a rapid process once the boiling point is reached, as it involves the entire liquid.
Occurrence Conditions
- Evaporation can occur under almost any condition, influenced by temperature, humidity, and air movement.
- Vaporization (boiling and sublimation) occurs under specific temperature and pressure conditions, requiring a threshold to be met or exceeded.
Evaporation Process
How Evaporation Occurs
Evaporation is a fascinating process that transforms a liquid into a gas without the need for boiling. This transformation unfolds through the following steps:
- Molecules Move: In any liquid, molecules are constantly moving. Some move faster than others.
- Energy Variation: Faster-moving molecules possess more energy compared to slower ones.
- Surface Escape: Occasionally, these fast-moving molecules hit the liquid’s surface and gain enough energy to overcome the attractive forces holding them in the liquid.
- Transition to Gas: Upon escaping, these molecules transition into the gas phase, becoming part of the surrounding air.
This series of steps underpins the seemingly simple act of water evaporating from a puddle or clothes drying on a line.
Role of Ambient Conditions
Ambient conditions, including temperature, humidity, and air movement, play a crucial role in the rate of evaporation:
- Temperature: Higher temperatures increase the energy of molecules, speeding up evaporation.
- Humidity: Lower humidity means the air can hold more water vapor, encouraging evaporation.
- Air Movement: Wind or air circulation helps remove vapor from the surface, making room for more evaporation.
Vaporization Process
How Vaporization Occurs
Vaporization, particularly boiling, occurs when a liquid reaches its boiling point and changes into gas. Here’s how the process unfolds:
- Heating: The liquid is heated to its boiling point.
- Bubble Formation: Energy causes bubbles of vapor to form within the liquid.
- Rising Bubbles: These bubbles rise to the surface and release the gas into the air.
This is the process observed when boiling water for tea or pasta.
Role of Heat
Heat plays a pivotal role in vaporization by providing the necessary energy for the phase change. In boiling:
- Energy Absorption: The liquid absorbs heat, which is then converted into kinetic energy for the molecules.
- Even Distribution: Heat must be distributed evenly throughout the liquid to ensure uniform boiling.
Practical Implications
Everyday Examples
The phenomena of evaporation and vaporization are integral to many everyday activities:
- Cooking: Boiling water to cook food is a daily application of vaporization.
- Cooling: Evaporative coolers use the principle of evaporation to cool air by passing it over water-soaked pads.
Industrial Applications
In industries, these processes have significant applications:
- Chemical Engineering: Distillation, a method used to separate mixtures, relies on the differences in boiling points, a direct application of vaporization.
- Food Processing: Freeze-drying food preserves it by removing water through sublimation, a form of vaporization.
Environmental Impact
Effect on Water Cycle
The water cycle, a critical environmental process, depends heavily on evaporation and vaporization:
- Evaporation from Oceans: The sun’s heat causes water from oceans and lakes to evaporate, forming clouds.
- Precipitation and Collection: This vapor then condenses, falls as precipitation, and collects in bodies of water, continuing the cycle.
Influence on Climate
Evaporation and vaporization also have a significant influence on climate:
- Regulating Temperatures: Evaporation cools surfaces, playing a crucial role in regulating Earth’s temperature.
- Cloud Formation: Vaporization contributes to cloud formation, which affects weather patterns and climate.
Frequently Asked Questions
Can both processes occur simultaneously?
Yes, both evaporation and vaporization can occur simultaneously under certain conditions. For example, on a hot day, water in a lake can evaporate from the surface at temperatures below boiling, while at the same time, water at the bottom, heated by the lake bed, might reach boiling and vaporize. This dual occurrence depends on environmental conditions and the distribution of heat within a liquid.
How does pressure affect these processes?
Pressure plays a critical role in both evaporation and vaporization. Lower atmospheric pressure decreases the boiling point of liquids, facilitating vaporization at lower temperatures. Similarly, evaporation increases under lower pressure as fewer air molecules are present to hinder the escape of liquid molecules into the gas phase. This is why water boils at lower temperatures on a mountain top compared to sea level.
Why is understanding these differences important?
Understanding the differences between evaporation and vaporization is crucial for various scientific and practical applications. It aids in predicting weather patterns, designing efficient cooling systems, and optimizing industrial processes such as distillation and desalination. Knowledge of these processes also helps in environmental conservation efforts by informing water management strategies and the study of climate change impacts.
Conclusion
The transition of matter from liquid to gas through evaporation and vaporization is a cornerstone of both natural phenomena and technological applications. These processes, governed by distinct physical conditions and principles, underscore the complexity and interconnectivity of the natural world and human innovation. Recognizing the nuances that differentiate evaporation from vaporization not only enriches our understanding of the physical world but also empowers us to harness these changes more effectively in various fields.
Our exploration of these phase changes illustrates the importance of precise scientific knowledge in addressing real-world challenges. As we continue to uncover the intricacies of evaporation and vaporization, we pave the way for advancements in technology, environmental science, and our daily lives, highlighting the endless curiosity and adaptability that drive human progress.