Chemical reactions are the backbone of countless processes, both in nature and industry, transforming substances through the breaking and forming of chemical bonds. These transformations are categorized based on their complexity and mechanism, into two primary types: elementary and non-elementary reactions. Each type plays a crucial role in the intricate dance of atoms and molecules, influencing everything from the synthesis of new materials to the metabolism within living organisms.
Elementary reactions are single-step processes where reactants convert to products in a single event, characterized by their simplicity and directness. In contrast, non-elementary reactions involve multiple steps, including the formation of intermediates before yielding the final products. This fundamental distinction affects how scientists study reaction mechanisms, predict outcomes, and harness chemical processes for various applications.
Understanding the difference between elementary and non-elementary reactions provides a clearer view of chemical kinetics and reaction mechanisms. This distinction is critical for chemists and engineers designing efficient reactions for industrial processes, pharmaceuticals, and environmental management. It highlights the importance of identifying reaction pathways, optimizing conditions for desired outcomes, and minimizing unwanted by-products.
Basic Concepts
Reaction Types
Definition of Chemical Reaction
A chemical reaction is a process where reactants transform into products. This transformation occurs through the breaking and forming of chemical bonds, leading to a change in the composition of substances. Chemical reactions underpin various natural and industrial processes, from photosynthesis in plants to the manufacturing of pharmaceuticals.
Overview of Elementary and Non-Elementary Reactions
Chemical reactions can be classified into two main types: elementary and non-elementary. Elementary reactions are simple, involving a single step where reactants convert directly to products. Non-elementary reactions are complex, consisting of multiple steps and often involving the formation of intermediates before reaching the final products.
Reaction Mechanisms
Explanation of Mechanisms
A reaction mechanism describes the step-by-step sequence in which a chemical reaction proceeds. It includes details about the transformation of reactants, the formation of intermediates, and the generation of products. Understanding a reaction’s mechanism is crucial for predicting the outcome of reactions and for designing new ones.
Role in Identifying Reaction Types
The mechanism of a reaction plays a key role in identifying whether it is elementary or non-elementary. Elementary reactions have a straightforward mechanism with no intermediates, while non-elementary reactions involve complex mechanisms with one or more intermediates. Analyzing the mechanism helps chemists understand the kinetics of a reaction, including how quickly it occurs and what factors influence its rate.
Elementary Reactions
Characteristics
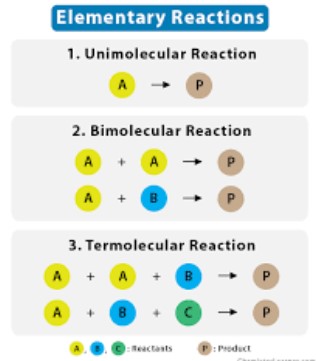
Single-step Process
Elementary reactions occur in a single step. All reactants directly form the products in one go, without any intermediate stages. This simplicity makes elementary reactions easier to analyze and predict compared to their non-elementary counterparts.
Molecularity Explanation
Molecularity refers to the number of molecules participating in the reaction. It is a key characteristic of elementary reactions and can be classified as unimolecular, bimolecular, or termolecular, depending on whether one, two, or three molecules are involved, respectively. Molecularity is directly related to the rate at which a reaction proceeds.
Examples
Common Examples of Elementary Reactions
- Unimolecular decomposition: A single molecule breaks down into two or more products. An example is the decomposition of ozone (O₃) into oxygen (O₂) and a single oxygen atom (O).
- Bimolecular reactions: Two molecules collide and react to form products. A classic example is the synthesis of water from hydrogen and oxygen gases.
- Termolecular reactions: These are less common due to the low probability of three molecules colliding at the same time. An example includes certain steps in the combustion of hydrocarbons.
Kinetics
Rate Laws for Elementary Reactions
The rate law for an elementary reaction can be directly derived from its molecularity. For a unimolecular reaction, the rate is proportional to the concentration of the single reactant. For a bimolecular reaction, the rate is proportional to the product of the concentrations of the two reactants.
Influence of Molecularity
Molecularity significantly influences the kinetics of an elementary reaction. A higher molecularity implies a lower probability of the required simultaneous collisions, generally leading to a slower reaction rate. Understanding this relationship helps chemists predict and control the speed of chemical processes.
Non-Elementary Reactions
Characteristics
Multi-step Processes
Non-elementary reactions are characterized by their multi-step processes. Unlike elementary reactions, they do not occur in a single action but through a series of intermediate steps. Each step has its own reactants, products, and unique rate law, making these reactions more complex and variable in nature.
Complex Mechanisms
The mechanisms of non-elementary reactions are intricate, involving several intermediate species and transitional states. These intermediates are not present in the starting reactants or final products but play crucial roles during the reaction. Understanding these mechanisms is essential for controlling and optimizing these reactions in various applications.
Examples
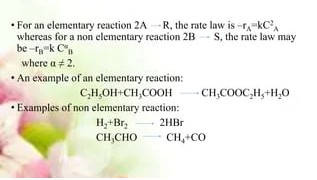
Common Examples of Non-Elementary Reactions
- Hydrocarbon combustion: A staple of energy production, the combustion of hydrocarbons in air involves numerous radical intermediates and a complex network of reactions.
- Photosynthesis: This natural process in plants involves multiple reaction steps to convert carbon dioxide and water into glucose and oxygen, using sunlight as energy.
- Polymerization: The creation of polymers from monomers involves several steps, including initiation, propagation, and termination phases.
Kinetics
Rate Laws for Non-Elementary Reactions
The rate laws for non-elementary reactions cannot be directly deduced from the stoichiometry of the reaction. Instead, they are determined empirically or derived from the detailed mechanism, considering the rates of the individual steps and the concentrations of intermediates.
Role of Intermediates
Intermediates play a pivotal role in non-elementary reactions, often determining the speed and pathway of the reaction. Their stability, concentration, and reactivity are key factors in the kinetics of these reactions, affecting both the rate and the outcome.
Comparison
Step Complexity
Comparison of Steps Involved
When comparing elementary and non-elementary reactions, the complexity of steps involved is a major distinction. Elementary reactions involve a single, straightforward step, while non-elementary reactions comprise multiple, interlinked steps. This difference significantly impacts the analysis, control, and prediction of reaction behaviors.
Reaction Rate
Differences in Determining Reaction Rates
Determining the reaction rates of non-elementary reactions is more challenging than for elementary reactions due to their complex mechanisms. While the rate of an elementary reaction can often be predicted from its molecularity, the rate of a non-elementary reaction requires a detailed understanding of all the intermediate steps and their kinetics.
Mechanism Clarity
Clarity and Predictability of Mechanisms
The mechanisms of elementary reactions are generally clear and predictable, owing to their single-step nature. In contrast, non-elementary reactions often have less clear and less predictable mechanisms, with multiple possible pathways and intermediates that can influence the course and outcome of the reaction.
Practical Implications
Industrial Applications
Impact on Chemical Manufacturing
Non-elementary reactions are pivotal in chemical manufacturing, where controlling the steps of a reaction can lead to improvements in yield, efficiency, and product purity. Industries rely on a deep understanding of these reactions to optimize conditions, reduce waste, and develop new processes for synthesizing materials and chemicals.
Research and Development
Influence on Scientific Research
The study of non-elementary reactions is a cornerstone of scientific research, especially in chemistry and biochemistry. Insights into reaction mechanisms and kinetics not only advance our fundamental understanding of chemical processes but also drive innovation in drug development, materials science, and energy technology.
Environmental Impact
Considerations in Environmental Chemistry
Understanding non-elementary reactions is crucial in environmental chemistry, where such reactions play a role in atmospheric processes, pollution control, and the degradation of contaminants. By manipulating these reactions, scientists and engineers can devise more effective strategies for environmental protection and remediation.
Frequently Asked Questions
What defines an elementary reaction?
An elementary reaction is defined by its single-step mechanism where reactants directly transform into products without any intermediate steps. These reactions are straightforward, with the reaction rate depending directly on the concentration of reactants, making them predictable and easy to model mathematically.
How do non-elementary reactions differ from elementary ones?
Non-elementary reactions differ in their complexity, involving multiple sequential or parallel steps to convert reactants into products. These reactions include intermediate species and may have varying rates for each step, complicating their kinetic modeling. Understanding these reactions requires detailed studies of their mechanisms.
Why is molecularity important in chemical reactions?
Molecularity refers to the number of molecules or atoms coming together to react in a single step of a chemical reaction. It’s crucial in defining the rate laws for elementary reactions, as it directly influences the reaction rate. Molecularity helps in distinguishing between unimolecular, bimolecular, and termolecular processes, each with distinct characteristics and kinetics.
How do reaction mechanisms impact industrial applications?
Reaction mechanisms provide vital insights into the steps involved in chemical reactions, helping industries to optimize conditions for maximum efficiency and yield. In pharmaceuticals, petrochemicals, and material science, understanding these mechanisms allows for the precise design of processes, reducing costs and enhancing product quality.
Conclusion
Distinguishing between elementary and non-elementary reactions is not just a matter of academic interest but a practical necessity in the realm of chemistry and its applications. This understanding enables scientists and engineers to predict reaction behaviors, control processes, and innovate in fields ranging from medicine to environmental protection. The clarity in mechanisms and reaction rates brought forth by this distinction guides the development of more efficient, sustainable, and cost-effective chemical processes.
The exploration of elementary and non-elementary reactions opens up a window to the molecular world, offering insights into the dynamic interactions that shape our physical reality. By mastering this knowledge, professionals in the field can contribute to advancements in technology, health, and environmental sustainability, marking the importance of chemical reactions in driving progress and innovation.
