Organic solvents are pivotal in the realm of chemistry, serving as the backbone for countless reactions and processes. Among these, diethyl ether and petroleum ether stand out for their widespread use in laboratories and industries. Each solvent carries unique properties and applications, making them indispensable in specific contexts. Yet, despite their shared nomenclature, diethyl ether and petroleum ether are fundamentally different substances.
Diethyl ether is a specific organic compound with a well-defined chemical structure, known for its role as an effective solvent and its application in various chemical syntheses. Petroleum ether, on the other hand, is not a single compound but a mixture of hydrocarbons primarily used for its solvent properties in extractions and purifications. The distinction lies not only in their chemical composition but also in their applications, safety profiles, and environmental impact.
This discussion will illuminate the chemical and practical differences between diethyl ether and petroleum ether. It will explore their unique characteristics, uses, and the considerations required when handling these substances. Such insights are essential for professionals and enthusiasts alike, ensuring the safe and effective application of these solvents in their respective domains.
Diethyl Ether Explained
Definition and Chemical Structure
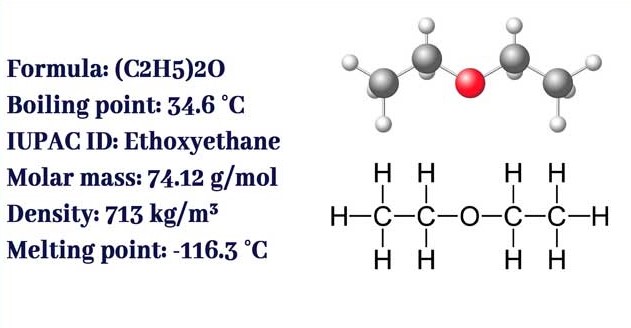
Diethyl ether, often referred to simply as ether, is a highly volatile, flammable organic compound used extensively as a solvent and in various chemical syntheses. Its chemical formula is C4H10O, and it is characterized by an oxygen atom connected to two ethyl groups. This structure is pivotal to its solvent properties, allowing it to dissolve a wide range of organic compounds. Diethyl ether’s distinct sweet odor is recognizable in laboratory settings, making it easy to identify.
Production Methods
The production of diethyl ether typically involves a two-step process:
- Synthesis of Ethanol: Ethanol is produced through the fermentation of sugars by yeasts or via chemical methods using ethylene.
- Dehydration of Ethanol: In this critical step, ethanol undergoes dehydration, usually in the presence of an acid catalyst, to form diethyl ether and water.
This method emphasizes the importance of controlling reaction conditions to achieve high yields of diethyl ether while minimizing by-products.
Common Uses and Applications
Diethyl ether has a plethora of uses, including:
- Solvent: Its ability to dissolve many organic compounds makes it invaluable in organic synthesis and extractions.
- Anesthetic: Historically, diethyl ether was used as a general anesthetic during surgeries.
- Starting Fluid: Its high volatility makes it an effective starting fluid in engines, especially under cold conditions.
Petroleum Ether Unveiled
Composition and Characteristics
Petroleum ether is not a single compound but a mixture of light hydrocarbons. It is obtained from the distillation of petroleum and consists mainly of alkanes. This mixture is characterized by its low boiling point and nonpolar nature, making it an excellent solvent for nonpolar substances. Unlike diethyl ether, petroleum ether does not have a specific molecular formula due to its variable composition.
How it is Obtained
Petroleum ether is produced through:
- Fractional Distillation of Petroleum: The process involves heating crude oil and collecting fractions at different boiling ranges. Petroleum ether is collected at the lower boiling range, separating it from heavier hydrocarbons.
Varied Uses in Industries
The nonpolar character of petroleum ether makes it suitable for:
- Extraction: It is used to extract oils and fats from natural sources.
- Purification: Its ability to dissolve certain compounds while leaving others undissolved is utilized in purification processes.
Key Differences
Chemical Structure and Composition
The primary difference between diethyl ether and petroleum ether lies in their chemical structure. Diethyl ether is a well-defined molecule with a clear structure, whereas petroleum ether is a mixture of hydrocarbons without a specific structure.
Boiling Point and Volatility
- Diethyl ether has a relatively low boiling point, which accounts for its high volatility.
- Petroleum ether encompasses a range of boiling points due to its mixture of components, but generally, it is designed to have a low boiling point for its solvent applications.
Safety and Health Risks
Both solvents pose risks, but their nature differs:
- Diethyl ether is highly flammable and can form explosive peroxides.
- Petroleum ether also presents fire hazards but does not form peroxides. However, its components can have varying toxicities.
Applications in the Lab and Industry
Their distinct properties dictate their applications:
- Diethyl ether is favored in chemical syntheses and as a laboratory solvent.
- Petroleum ether is predominantly used for extraction purposes in both laboratories and industries.
Chemical Structure Deep Dive
Diethyl Ether Structure
Molecular Formula
The molecular formula of diethyl ether is C4H10O, which is central to understanding its solvent capabilities.
Physical Properties
- Boiling Point: Around 34.6°C, contributing to its high volatility.
- Density: Slightly lighter than water, facilitating separations in liquid-liquid extraction processes.
Petroleum Ether Composition
Mixture Components
Petroleum ether consists of a blend of alkanes, each contributing to its overall nonpolar character and solvent properties.
Physical Characteristics
- Boiling Range: Typically between 30°C to 60°C, though this can vary based on the specific composition.
- Density: Generally lower than water, useful in certain types of extractions.
Production and Sourcing
Diethyl Ether Production
Synthesis Process
The synthesis of diethyl ether involves a simple yet crucial chemical reaction known as dehydration of ethanol. This process typically uses sulfuric acid as a catalyst and proceeds through a two-step mechanism:
- Ethanol Activation: Ethanol interacts with the acid, leading to the formation of an ethyl hydrogen sulfate intermediate.
- Ether Formation: A second ethanol molecule reacts with this intermediate, resulting in the production of diethyl ether and water.
This method emphasizes temperature control and the removal of water to drive the reaction forward, optimizing ether yield.
Sources of Raw Materials
The primary raw material for diethyl ether production is ethanol, which can be derived from:
- Fermentation of agricultural products like sugarcane, corn, and other starch-rich crops.
- Petroleum-based processes, where ethylene is hydrated to produce ethanol.
The choice of source depends on cost, availability, and purity requirements.
Petroleum Ether Obtaining
Distillation of Petroleum
Petroleum ether is sourced through the fractional distillation of crude oil. This complex process involves:
- Heating the crude oil to high temperatures in a distillation column.
- Separating the hydrocarbons based on their boiling points, with petroleum ether fractions collected at the lower temperatures.
Refining Process
After distillation, further refining steps may include:
- Hydrotreating to remove sulfur and improve purity.
- Blending different fractions to achieve desired solvent properties.
These steps ensure the consistency and quality of the petroleum ether.
Applications Contrast
In Laboratory Settings
Role in Synthesis
- Diethyl ether is favored for Grignard reactions, where its ability to dissolve reactants and stabilize the Grignard reagent is crucial.
- Petroleum ether, with its mix of nonpolar hydrocarbons, is less reactive and used for less sensitive reactions.
Solvent Capabilities
- Diethyl ether excels in dissolving a wide range of organic compounds, making it ideal for extractions and organic synthesis.
- Petroleum ether is used to dissolve nonpolar substances, such as oils and fats, due to its nonpolar nature.
Industrial Uses
Manufacturing Roles
- Diethyl ether is used in the manufacture of pharmaceuticals, particularly as a solvent and in the synthesis of active ingredients.
- Petroleum ether finds application in the production of paints and varnishes, where its solvent properties are utilized.
Extraction and Purification Processes
- Diethyl ether facilitates the extraction of natural products and the purification of chemicals in the pharmaceutical industry.
- Petroleum ether is widely used in the extraction of lipids from plant and animal sources due to its nonpolar character.
Safety and Handling
Diethyl Ether Risks
Flammability
Diethyl ether is highly flammable, with a low ignition point. It can form explosive peroxides when exposed to air for prolonged periods. Safe handling includes:
- Storing in airtight, dark glass bottles.
- Regularly checking for peroxide formation.
Health Hazards
Exposure to diethyl ether vapors can cause:
- Respiratory irritation.
- Dizziness and narcotic effects at high concentrations.
Proper ventilation and the use of personal protective equipment (PPE) are essential.
Petroleum Ether Safety
Precautions
Handling petroleum ether requires precautions to minimize:
- Inhalation of vapors, which can be toxic.
- Skin contact, leading to irritation.
Disposal and Environmental Impact
Proper disposal involves:
- Recycling or incineration in designated facilities.
- Avoiding release into the environment to prevent water and soil pollution.
Environmental Impact
The environmental impact of chemical solvents like diethyl ether and petroleum ether is a subject of increasing concern. Both of these substances, while invaluable in various industrial and laboratory settings, carry significant environmental footprints. Their production, usage, and disposal need to be managed carefully to mitigate negative effects on the environment.
Diethyl Ether Concerns
Emissions and Pollution
Diethyl ether is known for its volatility, a property that, while useful in certain applications, contributes to its environmental impact. When released into the atmosphere, diethyl ether vapors can contribute to air pollution. These emissions not only pose a risk to air quality but also have the potential to contribute to the formation of ground-level ozone, a key component of smog.
- Mitigation Strategies:
- Use chemical fume hoods to capture vapors during laboratory work.
- Implement tight-sealing containers for storage and waste to minimize accidental releases.
Petroleum Ether Impact
Sustainability Issues
The production of petroleum ether, derived from crude oil, brings to light several sustainability concerns. The reliance on fossil fuels for its production not only depletes these non-renewable resources but also contributes to greenhouse gas emissions during extraction, refining, and transportation processes.
- Alternatives and Solutions:
- Exploration of bio-based solvents as sustainable alternatives.
- Investment in energy-efficient refining technologies to reduce carbon footprint.
Regulation and Control
The handling, storage, and disposal of both diethyl ether and petroleum ether are subject to strict regulations aimed at protecting both human health and the environment. These regulations vary by country but generally include guidelines on:
- Air Quality Management: Limiting emissions of volatile organic compounds (VOCs) to prevent air pollution.
- Waste Disposal: Ensuring that solvent waste is treated or disposed of in a manner that minimizes environmental impact.
- Workplace Safety: Reducing the risk of exposure to workers and the surrounding community.
Frequently Asked Questions
What is Diethyl Ether Used For?
Diethyl ether is widely used as an anesthetic, a high-purity solvent in chemical reactions, and in the production of other chemicals. Its volatility and ability to dissolve a wide range of substances make it invaluable in laboratories for conducting extractions and as a solvent in Grignard reactions, among other applications.
How is Petroleum Ether Different from Diethyl Ether?
Petroleum ether and diethyl ether differ primarily in their composition and properties. Petroleum ether is a mixture of light hydrocarbons and is used as a nonpolar solvent, mainly in the extraction of oils and fats, whereas diethyl ether is a single, highly volatile organic compound with both anesthetic and solvent applications.
Are Diethyl Ether and Petroleum Ether Interchangeable?
No, diethyl ether and petroleum ether are not interchangeable. Their differing chemical compositions and properties mean they are suited to different applications. Diethyl ether’s high volatility and specific reactivity make it suitable for specialized chemical syntheses, while petroleum ether’s nonpolar nature makes it ideal for extracting nonpolar compounds.
What are the Safety Concerns with Diethyl Ether and Petroleum Ether?
Both solvents pose significant safety concerns; however, their risks differ. Diethyl ether is highly flammable, with a risk of peroxide formation upon prolonged exposure to air, requiring careful handling and storage. Petroleum ether, while also flammable, presents a lower risk of peroxide formation but requires ventilation to manage its potent fumes and minimize inhalation risks.
Conclusion
In concluding our exploration of diethyl ether and petroleum ether, it becomes clear that while both solvents are foundational in the chemical and industrial fields, their differences are profound. Diethyl ether, with its specific chemical structure, serves unique roles in both laboratory settings and the medical field. Petroleum ether, a blend of hydrocarbons, finds its niche in the extraction and purification of compounds, highlighting the importance of choosing the right solvent for the task at hand.
Understanding these solvents’ distinct characteristics enables practitioners to harness their properties effectively while mitigating associated risks. As we navigate their uses and applications, it’s essential to appreciate not just their utility but also the responsibility that comes with handling such powerful chemical tools.
