Cyanide and nitrile, two compounds widely recognized for their significance in various industrial and environmental contexts, often get mentioned together yet serve distinct purposes and carry different implications. Both have unique properties and applications that make them invaluable in their respective fields, from manufacturing to environmental management. Despite their shared presence in the chemical industry, the differences between them are substantial and worth exploring.
Cyanide is a highly toxic chemical compound known for its potential to cause lethal harm to humans and the environment. In contrast, nitrile refers to a group of organic compounds characterized by a cyano group attached to an alkyl group, widely used in the production of synthetic rubbers and plastics. Understanding the distinction between these two is crucial for their safe handling, usage, and the mitigation of associated risks.
The topic at hand involves not just a comparison of chemical structures but also an in-depth look at how each compound interacts with living organisms and the environment. Cyanide, with its notorious reputation, and nitrile, known for its versatility in the manufacturing sector, play contrasting roles in modern industry and environmental health. Recognizing these differences is essential for professionals in chemistry, environmental science, and related fields, as well as for a general audience interested in the impact of these substances on our world.
Cyanide Explained
Definition and Characteristics
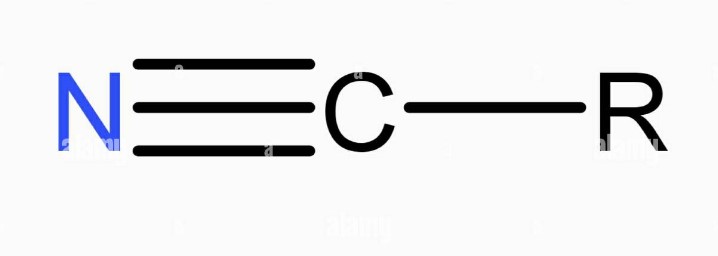
Cyanide is a term that often evokes images of danger and toxicity. At its core, cyanide is a chemical compound that consists of a carbon atom triple-bonded to a nitrogen atom (CN). This simple structure belies its potent effects; cyanide can interfere with the body’s ability to use oxygen, making it extremely toxic to humans and animals.
Common Sources and Applications
Cyanide’s notoriety doesn’t stop it from being used across various industries. Its applications are as diverse as they are critical. In gold mining, cyanide is a key component in extracting gold from ore. The chemical industry utilizes cyanide for the production of synthetic fibers and plastics. Despite its dangers, cyanide’s effectiveness in these processes makes it indispensable.
Health and Environmental Impacts
The impact of cyanide on health and the environment cannot be understated. Exposure to cyanide can lead to symptoms ranging from headaches and dizziness to seizures and even death. Environmentally, cyanide spills can devastate aquatic ecosystems, killing fish and other wildlife. Thus, strict regulations govern its use and disposal.
Nitrile Overview
Definition and Key Properties
Moving to a less notorious but equally fascinating compound, nitrile refers to organic compounds containing the cyano group (-C≡N), bound to an alkyl group. Nitriles are known for their stability and resistance to harsh chemicals, making them incredibly useful in various applications.
Usage in Various Industries
Nitriles find their place in multiple sectors due to their versatile properties. Nitrile rubber, for example, is a material of choice for making gloves, gaskets, and hoses, prized for its resistance to oils and chemicals. Additionally, the pharmaceutical industry uses nitriles in the synthesis of drugs, showcasing their broad utility.
Safety and Environmental Considerations
While nitriles are less toxic than cyanide, they are not without their hazards and environmental considerations. Handling nitrile compounds requires care, as some can be irritants or have other health effects. Environmentally, the production and disposal of nitrile-based products, like synthetic rubber, demand responsible management to prevent pollution and reduce waste.
Chemical Structures
Cyanide Ion Composition
The cyanide ion (CN−) is a simple yet potent entity. Its structure features a carbon atom and a nitrogen atom connected by a triple bond, giving it a linear shape. This configuration is key to its reactivity and its ability to form complexes with metals, which is exploited in gold mining.
Nitrile Group Structure
In contrast, the nitrile group consists of a carbon triple-bonded to nitrogen, but here, the carbon is also connected to an alkyl group. This structure imparts nitriles with their characteristic chemical resistance and stability, distinguishing them from the highly reactive cyanide ion.
Visual Comparison
A visual comparison of cyanide and nitrile structures reveals their similarities and differences. Both have the carbon-nitrogen triple bond, but the presence of an alkyl group in nitriles changes their properties and applications significantly.
Applications Contrast
Cyanide in Mining and Manufacturing
Cyanide’s role in mining is particularly significant. It allows for the efficient extraction of gold from ore through a process called cyanidation, where cyanide leaches out the precious metal. In manufacturing, cyanide compounds are used to make nylons and acrylics, demonstrating its versatility.
Nitrile in Synthetic Materials
Nitrile shines in the production of synthetic materials. Nitrile rubber is a standout, offering unmatched resistance to oils and chemicals, which makes it ideal for automotive and industrial applications. This material’s durability under adverse conditions underscores the value of nitriles in modern manufacturing.
Unique Roles in Different Sectors
The roles of cyanide and nitrile in their respective sectors highlight their unique characteristics and indispensability. While cyanide is crucial for gold extraction and the synthesis of various chemicals, nitriles provide the backbone for resilient synthetic rubbers and plastics. Their applications, though different, underscore the diverse capabilities of these compounds in industry and beyond.
Health Risks
Acute and Chronic Effects of Cyanide
Cyanide poses significant health risks upon exposure, with effects ranging from acute to chronic. Acute exposure can lead to symptoms such as dizziness, headache, and in severe cases, respiratory failure and death. Chronic exposure, though less immediate, can result in cardiovascular and neurological issues, underlining the necessity for stringent safety measures.
Nitrile Exposure and Toxicity
Exposure to certain nitrile compounds can also have health implications, though generally less severe than cyanide. Symptoms might include skin and eye irritation, and prolonged exposure can lead to more serious conditions depending on the specific compound and level of exposure. The risk varies with the type of nitrile and the exposure scenario.
Safety Measures and Antidotes
To mitigate these risks, comprehensive safety measures and antidotes are essential. For cyanide:
- Use personal protective equipment (PPE)
- Install proper ventilation systems
- Train workers on emergency procedures
- Have cyanide antidotes readily available, such as oxygen therapy and sodium thiosulfate.
For nitriles:
- Employ similar PPE
- Ensure proper handling and storage
- Provide training on spill management.
Environmental Impact
Cyanide Pollution Sources
Cyanide’s environmental impact primarily comes from industrial processes like mining, where it can leach into water sources, posing a threat to aquatic life. It’s also a concern in manufacturing settings, where spills and disposals contribute to pollution.
Nitrile Degradation and Recyclability
Nitrile compounds vary in their environmental persistence. While some degrade over time, others remain, contributing to pollution. However, advancements in recycling and biodegradation technologies are improving the outlook for nitrile rubber and other products, reducing their environmental footprint.
Mitigating Environmental Risks
Mitigating the environmental risks of these chemicals involves:
- Proper waste management practices
- Investing in cleaner production technologies
- Implementing spill response strategies
- Enhancing recycling efforts.
Legal and Safety Regulations
Cyanide Regulatory Framework
The handling and use of cyanide are subject to a rigorous regulatory framework to protect workers and the environment. Regulations cover aspects such as transport, storage, use, and disposal, with compliance being monitored by governmental bodies.
Nitrile Handling and Disposal Guidelines
Similar regulations apply to nitriles, with guidelines ensuring safe handling, storage, and disposal to prevent exposure and environmental contamination. Compliance challenges can arise, however, due to varying international standards and the broad range of nitrile compounds used across industries.
Compliance Challenges
Staying compliant with these regulations can be challenging, especially for multinational companies. Differences in local laws, the complexity of guidelines, and the need for continuous training and awareness contribute to these challenges.
Industrial Uses
Gold Extraction and Cyanide
In the mining industry, cyanide is essential for gold extraction. It allows for the efficient processing of low-grade ores, making gold mining economically viable. However, the environmental and health risks associated with cyanide use have spurred research into alternative methods.
Nitrile Rubber Production
Nitrile rubber is a critical material in many applications due to its resistance to oils and chemicals. Its production involves the polymerization of nitriles, and advancements in production techniques continue to improve its properties and applications.
Innovations and Future Trends
Innovation is key to reducing the risks associated with cyanide and nitriles. For cyanide, research into less toxic alternatives for gold extraction is ongoing. In the case of nitriles, developments in biodegradable nitriles and improved recycling methods are trends that promise a more sustainable future.
Public Perception
Historical Incidents Involving Cyanide
Cyanide has been involved in numerous historical incidents, from industrial accidents to its infamous use in suicides and murders. These events have shaped public perception, associating cyanide with danger and toxicity.
Nitrile as a Safer Alternative
Compared to cyanide, nitriles are generally perceived as safer, especially in consumer products like gloves and seals. This perception is supported by the lower toxicity of nitriles and their essential role in everyday items.
Impact on Consumer Choices
Public perception influences consumer choices. Concerns over cyanide’s impact have led consumers and industries to seek safer, more environmentally friendly alternatives. Similarly, the demand for durable and safe nitrile-based products continues to grow, reflecting a preference for materials that balance performance with health and environmental considerations.
Frequently Asked Questions
What is Cyanide?
Cyanide is a chemical compound composed of carbon and nitrogen. It is known for its high toxicity to humans and animals when ingested or inhaled. Cyanide compounds are used in various industrial processes, including mining and electroplating, but they require careful handling due to their potential health risks.
How is Nitrile Used in Industry?
Nitrile is primarily used in the production of nitrile rubber, a synthetic material found in many products like gloves, hoses, and seals. Thanks to its resistance to oils, fuels, and other chemicals, nitrile rubber is invaluable in automotive and aerospace industries, providing durability and longevity in harsh conditions.
Can Cyanide and Nitrile Affect the Environment?
Yes, both cyanide and nitrile can impact the environment, but in different ways. Cyanide, due to its toxicity, poses a significant threat to aquatic life and water quality if not properly managed. Nitrile compounds, while less toxic, can contribute to plastic pollution and are a concern in waste management and recycling efforts.
Are There Safety Regulations for Handling Cyanide and Nitrile?
Both cyanide and nitrile are subject to safety regulations to prevent exposure and environmental contamination. These regulations include guidelines for safe handling, storage, and disposal, as well as emergency response measures for spills or exposure incidents. Compliance with these rules is critical for protecting workers and the environment.
Conclusion
The distinction between cyanide and nitrile extends beyond their chemical composition to encompass their applications, health risks, and environmental impacts. Cyanide, with its potent toxicity, demands rigorous safety measures and awareness to prevent harm to humans and ecosystems. On the other hand, nitrile offers a range of uses in various industries due to its chemical resilience, yet it also necessitates responsible management to mitigate its environmental footprint.
Recognizing and understanding these differences is not just an academic exercise but a necessity for the safe and sustainable use of these compounds. As our world continues to rely on chemicals for a myriad of applications, the knowledge and precautions surrounding cyanide and nitrile serve as a crucial reminder of our responsibility towards health, safety, and environmental stewardship.