Crystal Field Theory (CFT) offers a remarkable framework for understanding the behavior of transition metal complexes. It revolves around the idea that the spatial arrangement of ligands around a central metal atom influences the energy of the d-orbitals. This fundamental concept has profoundly impacted the field of coordination chemistry, allowing scientists to predict the properties of complex molecules with surprising accuracy.
The difference between Crystal Field Stabilization Energy (CFSE) and splitting energy lies at the heart of CFT. CFSE is the energy released when d-orbitals split in the presence of ligands, stabilizing the complex. Splitting energy, on the other hand, refers to the energy required to overcome the repulsion between electrons in the newly formed d-orbital levels. These concepts are crucial for predicting the color, magnetic properties, and reactivity of transition metal complexes.
Understanding CFSE and splitting energy is essential for chemists and materials scientists. These concepts not only explain the underlying principles of complex formation but also provide insights into the design of new materials with desired properties. From the vibrant colors of gemstones to the catalytic activities of industrial processes, the principles of CFSE and splitting energy play a pivotal role in explaining the fascinating world of coordination chemistry.
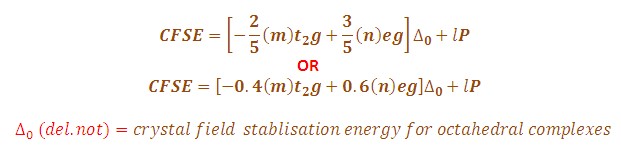
Crystal Field Theory
Explanation of Ligand Field Interaction
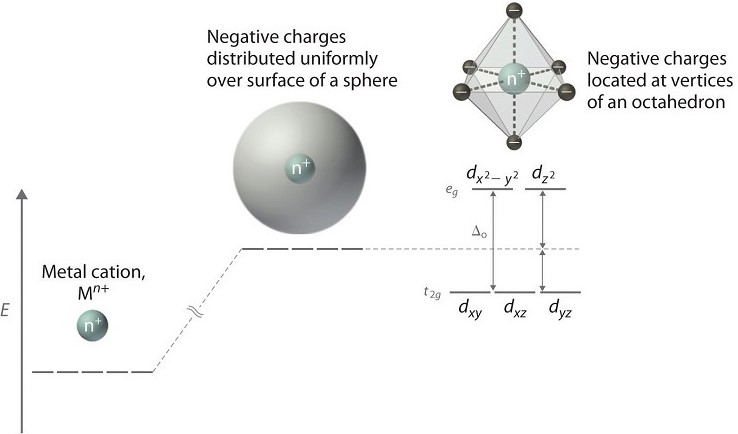
Crystal Field Theory (CFT) is a quantum mechanical model describing the effect of ligands on the distribution of electrons in the d-orbitals of transition metal ions. When ligands approach the central metal ion, the degenerate (equally energetic) d-orbitals split into different energy levels due to the electrostatic interactions between the ligands’ electrons and the d-electrons of the metal.
This interaction depends on the nature of the ligands, their arrangement around the metal, and the overall geometry of the complex. It’s crucial to understand that the orientation of d-orbitals (d_x, d_y, d_z, etc.) in relation to the incoming ligands determines the extent of energy splitting.
Role in Transition Metal Complexes
CFT explains various physical and chemical properties of transition metal complexes such as color, magnetism, and reactivity. It helps in understanding why certain complexes are more stable than others and predicts the type of isomerism they exhibit. For chemists, CFT is a tool to decipher the complex behaviors of metal ions when they interact with a set of ligands, guiding the synthesis of new compounds with desired properties.
Energy Splitting
Definition and Causes
Energy splitting in the context of CFT refers to the separation of the d-orbital energies into different levels upon coordination with ligands. The primary cause is the electrostatic repulsion between the electrons in the ligands and the electrons in the d-orbitals of the central metal ion. This repulsion varies based on the spatial arrangement of the ligands, leading to different patterns of energy splitting.
d-Orbital Splitting in Complexes
Octahedral Complexes
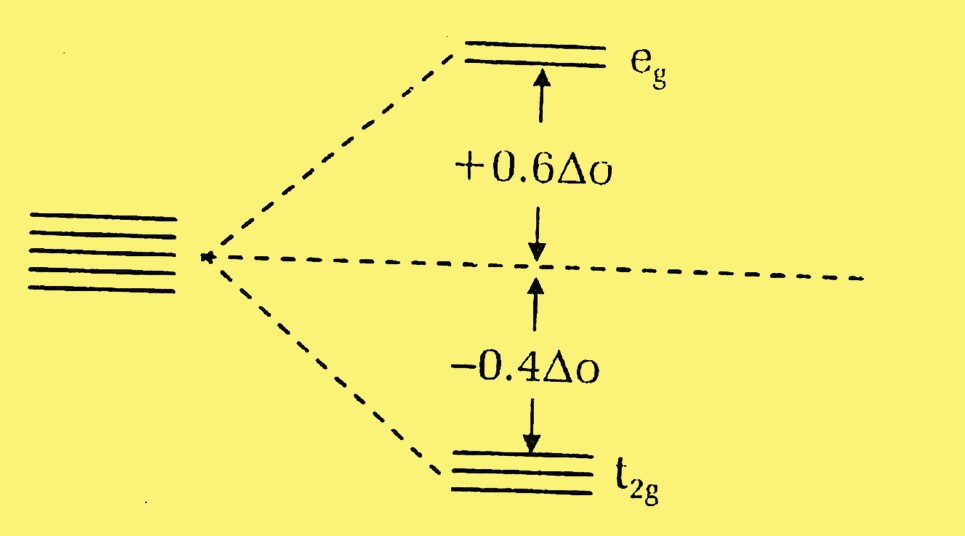
In an octahedral complex, where six ligands symmetrically surround the metal ion, the d-orbitals split into two sets: t_2g (lower energy) and e_g (higher energy). The difference in energy between these sets is termed the splitting energy (Δ_oct).
Tetrahedral Complexes
Tetrahedral complexes exhibit a reverse pattern compared to octahedral, with only four ligands positioned at the corners of a tetrahedron. Here, the e_g orbitals are lower in energy than the t_2g, and the splitting energy (Δ_tet) is generally smaller than in octahedral complexes.
Square Planar Complexes
Square planar complexes, often formed by d^8 metal ions, show a more complex splitting pattern, with four ligands positioned in a plane. This leads to a significant splitting where the d_xy orbital remains non-bonding, and the energy levels are quite distinct compared to octahedral and tetrahedral geometries.
Stabilization Energy
Concept of Crystal Field Stabilization Energy (CFSE)
Crystal Field Stabilization Energy (CFSE) is the energy released as a result of d-orbital splitting when ligands coordinate to a metal ion. It is a measure of the stability that a complex gains due to the specific arrangement of its ligands. The more negative the CFSE, the more stable the complex.
Calculation and Factors Affecting CFSE
CFSE can be calculated by considering the electronic configuration of the metal ion, the geometry of the complex, and the placement of electrons in the split d-orbitals. Key factors affecting CFSE include:
- The charge on the metal ion
- The nature of the ligands (weak field vs. strong field)
- The geometry of the complex (octahedral, tetrahedral, square planar)
CFSE and Splitting Energy
Defining CFSE
CFSE is defined mathematically as the difference in energy before and after the d-orbitals of a metal ion split in the presence of ligands. It reflects how much more stable a complex is compared to when its d-electrons were degenerate.
Mathematical Expression
For an octahedral complex, CFSE can be calculated using the formula:
CFSE=(0.4×��2�−0.6×���)×Δ���+�CFSE=(0.4×nt2g−0.6×neg)×Δoct+P
where ��2�nt2g and ���neg are the number of electrons in the t_2g and e_g orbitals, respectively, Δ���Δoct is the octahedral splitting energy, and �P is the pairing energy.
Dependence on Ligand and Geometry
The magnitude of CFSE is highly dependent on the strength of the ligand (as outlined in the spectrochemical series) and the complex’s geometry. Strong field ligands and certain geometries (like octahedral) tend to increase CFSE, stabilizing the complex further.
Splitting Energy Explained
Nature of d-Orbital Splitting
The nature of d-orbital splitting can be viewed as a consequence of the electrostatic field created by the ligands surrounding the metal ion. The spatial arrangement of the d-orbitals with respect to the ligands dictates the pattern and magnitude of the splitting.
Spectrochemical Series and its Impact
The spectrochemical series ranks ligands based on their ability to split the d-orbital energies. Ligands at one end of the series cause a small split (weak field ligands), whereas those at the other end cause a large split (strong field ligands). This series is pivotal in predicting the color, magnetic properties, and overall stability of coordination compounds.
Key Differences Between CFSE and Splitting Energy
Understanding the distinction between Crystal Field Stabilization Energy (CFSE) and splitting energy illuminates the intricate dance of electrons in coordination chemistry. These concepts, while related, focus on different aspects of the interactions within transition metal complexes.
Contrast in Definitions
CFSE and splitting energy serve as fundamental concepts in coordination chemistry, each illuminating a unique aspect of ligand-metal interactions.
- CFSE is the stabilization energy a complex gains when ligands disrupt the degeneracy of d-orbitals, leading to lower energy states.
- Splitting energy, on the other hand, refers to the energy difference between the higher and lower sets of split d-orbitals.
This distinction underscores the complementary nature of these concepts, providing a comprehensive understanding of complex formation and stability.
Calculation Variance
Calculating CFSE and splitting energy involves different approaches and considerations:
- CFSE calculation requires knowledge of the electron configuration of the metal ion, the geometry of the complex, and the distribution of electrons among the split d-orbitals.
- Splitting energy is determined by measuring the energy absorbed or emitted when an electron transitions between split d-orbitals, often observed through spectroscopic techniques.
These calculations not only reveal the inherent stability and properties of complexes but also underscore the nuanced relationship between electronic structure and observed chemical behavior.
Impact on Complex Stability
The roles of CFSE and splitting energy in influencing complex stability are profound:
- A higher CFSE indicates a more stable complex, as it reflects a greater release of energy due to the efficient arrangement of electrons.
- Splitting energy impacts stability indirectly through its influence on ligand field strength and the likelihood of electronic transitions.
Understanding these impacts is crucial for designing complexes with desired stability and reactivity profiles, highlighting the intricate balance of forces at play in coordination chemistry.
Influence of Ligands and Geometry
Ligands and geometry play pivotal roles in determining CFSE and splitting energy:
- Strong field ligands and certain geometries (e.g., octahedral) increase CFSE, enhancing stability.
- The choice of ligands and the complex’s geometry also affect splitting energy, altering the energy required for electronic transitions and, consequently, the complex’s color and magnetic properties.
This interplay underscores the importance of strategic ligand and geometry selection in the synthesis and application of coordination compounds.
Real-World Applications
The principles of CFSE and splitting energy find application across a spectrum of scientific endeavors, from the synthesis of vibrant pigments to the development of groundbreaking pharmaceuticals.
Color of Compounds
The relationship between splitting energy and the color of compounds is a direct consequence of electronic transitions:
- The absorption of visible light corresponds to the energy difference between split d-orbitals, with the observed color being complementary to the absorbed wavelength.
- Manipulating splitting energy through ligand selection and complex geometry enables the design of compounds with specific colors, essential for dyes and pigments.
Magnetic Properties
CFSE directly influences the magnetic behavior of complexes by dictating the distribution of electrons among d-orbitals:
- High CFSE can lead to low-spin configurations, affecting the compound’s magnetic susceptibility.
- Understanding this relationship is crucial for designing materials with desired magnetic properties, including applications in data storage and spintronics.
Catalysis and Drug Design
The principles of CFSE and splitting energy are instrumental in catalysis and drug design:
- Catalysts with optimized CFSE can exhibit enhanced activity and selectivity, crucial for industrial and environmental processes.
- In drug design, these principles guide the development of metal-based therapeutics, leveraging specific ligand interactions to target biological systems effectively.
Challenges and Considerations
While the concepts of CFSE and splitting energy offer valuable insights, they also present challenges and considerations for further exploration.
Limitations of CFSE and Splitting Energy Models
The models for CFSE and splitting energy, though powerful, have limitations:
- They often rely on idealized assumptions that may not fully account for complex real-world interactions.
- Recognizing these limitations is essential for advancing our understanding and application of these concepts in more complex systems.
Experimental Determination
Determining CFSE and splitting energy experimentally involves sophisticated techniques:
- Spectroscopic methods, such as UV-Vis and EPR spectroscopy, are commonly employed to measure splitting energy and infer CFSE.
- These techniques require meticulous calibration and interpretation, highlighting the importance of experimental rigor in the study of coordination chemistry.
Frequently Asked Questions
What is Crystal Field Theory?
Crystal Field Theory explains how the arrangement of ligands around a central metal ion affects the energy levels of the metal’s d-electrons. This interaction leads to the splitting of d-orbital energy levels, impacting the complex’s properties, such as color and magnetic behavior.
How is CFSE calculated?
Crystal Field Stabilization Energy (CFSE) is calculated based on the difference in energy before and after the d-orbitals of a metal ion split in the presence of ligands. This involves considering the specific geometry of the complex (e.g., octahedral or tetrahedral) and the distribution of electrons in the split d-orbitals.
Why do d-orbitals split in energy?
D-orbitals split in energy in the presence of ligands due to the electrostatic repulsion between the d-electron cloud of the metal ion and the negative charge or dipole of the ligands. This repulsion is not uniform for all d-orbitals, leading to energy splitting.
How does splitting energy relate to the color of compounds?
The splitting energy determines the wavelength of light absorbed by a complex, which, in turn, influences its color. Compounds absorb light in the visible region if the energy difference between the split d-orbitals matches the energy of the visible light, leading to the complementary color being observed.
Conclusion
The concepts of Crystal Field Stabilization Energy and splitting energy are fundamental pillars of coordination chemistry. They offer profound insights into the behavior of transition metal complexes, enabling scientists to predict and manipulate the physical and chemical properties of these compounds. The understanding of these principles not only enriches our knowledge of chemistry but also opens avenues for innovation in material science, catalysis, and pharmaceuticals.
As we continue to explore the intricacies of molecular interactions, the study of CFSE and splitting energy will remain a cornerstone in the development of new technologies and solutions. The journey through the microscopic world of d-orbitals reveals the complexity and beauty of the universe’s building blocks, reminding us of the endless possibilities that await discovery.
