Organic chemistry is fundamental to our understanding of countless substances that are essential in various industries, from pharmaceuticals to manufacturing. Among these substances, cresol and phenol stand out due to their widespread use and chemical significance. Both compounds belong to the phenol family, but their properties and applications vary significantly.
Cresol and phenol are aromatic organic compounds with differing molecular structures and uses. Cresol, also known as methylphenol, includes several isomers that make it versatile in medical and industrial applications. In contrast, phenol itself is simpler but equally indispensable, utilized in everything from the production of plastics to pharmaceuticals.
These compounds not only differ in structure and use but also in their production methods, health implications, and environmental impacts. Despite their similarities, the distinct characteristics of cresol and phenol dictate their roles in various sectors, making a clear understanding of their differences crucial for professionals across multiple industries.
Basic Definitions
Cresol Overview
Definition and Structure
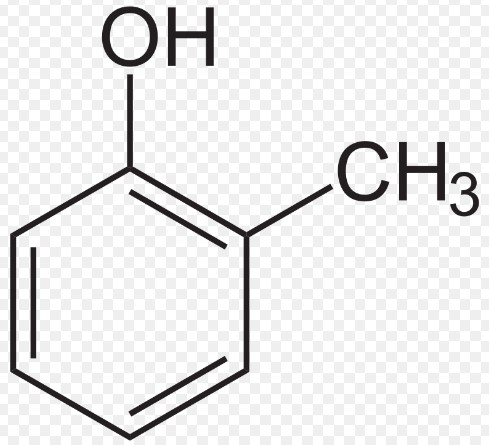
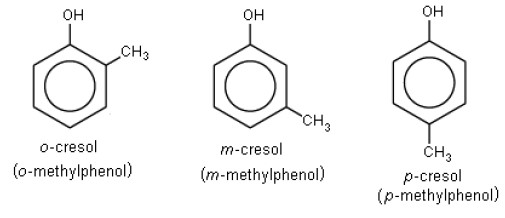
Cresol, also known as methylphenol, is an organic compound belonging to the family of phenols. The molecular structure of cresol includes a benzene ring attached to a hydroxyl group (OH) and a methyl group (CH₃). This structure varies slightly among its three main isomers: ortho-cresol (o-cresol), meta-cresol (m-cresol), and para-cresol (p-cresol), where the positions of the hydroxyl and methyl groups on the benzene ring differ.
Types and Sources
Cresols are primarily found in three types based on the position of the methyl group:
- Ortho-cresol (2-methylphenol): Often used in disinfectants and fumigants.
- Meta-cresol (3-methylphenol): Commonly used in antiseptics and certain chemical intermediates.
- Para-cresol (4-methylphenol): Frequently found in the manufacture of plastics and pesticides.
Sources of cresols include coal tar and petroleum. They can also be synthesized chemically from phenol through methylations or as byproducts in the production of coke from coal.
Phenol Overview
Definition and Structure
Phenol itself is a simple aromatic compound with a structure consisting of a benzene ring bonded to a hydroxyl group. It is known for its slightly acidic properties and is capable of forming weak bonds with water molecules.
Common Sources
Phenol is typically produced from petroleum and is also found naturally in low concentrations in various environmental settings, including as a decomposition product of organic materials. Industrial synthesis remains the dominant source, with large-scale production processes specifically designed to meet global demand.
Chemical Properties
Cresol Properties
Physical State, Boiling and Melting Points
Cresols are colorless to pale yellow liquids at room temperature. The boiling points of cresols range from 190°C to 203°C, and their melting points are between 30°C and 36°C, varying slightly among the isomers.
Solubility and Reactivity
Cresols are soluble in water, alcohol, and ether. They react with bases to form cresolates, which are more soluble and can be used in various applications. Their chemical reactivity also includes reactions with nitric acid and other oxidizing agents, which can produce more complex phenolic compounds.
Phenol Properties
Physical Characteristics
Phenol is typically a white crystalline solid that becomes pink when exposed to light due to oxidation. It has a characteristic sweet and tarry odor with a melting point of about 40.5°C and a boiling point of 181.75°C.
Chemical Behavior in Reactions
Phenol is reactive towards electrophilic aromatic substitution. It can undergo halogenation, nitration, and sulfonation, making it a versatile precursor in the synthesis of more complex chemicals. Phenol’s acidity allows it to form phenoxide ions when reacted with bases, which are important in further chemical reactions.
Production Methods
Cresol Synthesis
Industrial Processes
Industrial production of cresol typically involves the alkylation of phenol with methanol in the presence of a catalyst. This method can selectively produce different isomers of cresol based on the specific conditions and catalysts used.
Lab-scale Synthesis
On a smaller scale, cresols can be synthesized by distilling coal tar or through the hydrolysis of chlorotoluenes. These methods allow for the production of cresols in controlled environments, useful for research and development purposes.
Phenol Synthesis
Cumene Process
The Cumene process is the most widely used method for the industrial production of phenol. It involves the following steps:
- Alkylation: Cumene is produced by the alkylation of benzene with propylene.
- Oxidation: Cumene is oxidized to cumene hydroperoxide.
- Acid-catalyzed cleavage: The hydroperoxide is then cleaved in the presence of an acid catalyst to form phenol and acetone.
Other Production Techniques
Other methods include the direct oxidation of benzene or the sulfonation of benzene followed by base fusion. These methods are less common but still relevant in producing phenol for specific industrial needs.

Applications
Uses of Cresol
Medical and Antiseptic Solutions
Cresol is a key ingredient in various medical and antiseptic solutions due to its bactericidal properties. It is used in:
- Hospital disinfectants: Effective in killing bacteria and viruses on surfaces.
- Antiseptic creams: Used to treat skin infections and sanitize minor wounds.
Cresol’s ability to disrupt microbial cell walls makes it a valuable component in the healthcare sector, ensuring sterile environments and preventing the spread of infectious diseases.
Pesticides and Resin Manufacturing
In agriculture, cresol serves as a base chemical in the formulation of pesticides. Its effectiveness in pest control helps protect crops and maintain food supply integrity. Additionally, cresol is utilized in the manufacturing of resins and plastics, where it acts as a precursor to various high-performance materials used in automotive and aerospace industries.
Uses of Phenol
Production of Plastics and Drugs
Phenol plays a crucial role in the plastics industry, particularly in the production of:
- Polycarbonate plastics: Known for their strength and durability.
- Phenolic resins: Used in insulation, laminates, and molded products.
In the pharmaceutical sector, phenol is essential for synthesizing active pharmaceutical ingredients and excipients, contributing to the development of various medications.
Role in Cosmetic Products
Phenol is also significant in the cosmetics industry. It is used in:
- Skin peels: Helps in treating acne and reducing fine lines.
- Hair dyes: Acts as a stabilizing agent.
Its properties ensure safety and effectiveness in products intended for personal care and beauty.
Health and Environmental Impact
Cresol Safety Concerns
Toxicity Levels and Exposure Risks
Cresol’s toxicity can pose serious health risks if mishandled. Exposure can result from inhalation, ingestion, or direct skin contact, leading to:
- Respiratory issues
- Skin burns
- Potential systemic toxicity
Proper handling and adherence to safety guidelines are imperative to mitigate these risks.
Environmental Effects
Cresol can affect the environment if not properly managed. Its release into water bodies can lead to:
- Aquatic toxicity
- Disruption of local ecosystems
Efforts to minimize environmental exposure are critical in industries using cresol.
Phenol Safety Concerns
Health Hazards Associated with Phenol
Phenol is toxic and can cause severe health issues upon exposure, including:
- Chemical burns
- Respiratory problems
- Systemic organ damage
Understanding and controlling exposure levels are essential for safe phenol use.
Environmental Considerations
Phenol’s impact on the environment is notable, especially when it enters water systems. It can cause:
- Aquatic life toxicity
- Long-term soil contamination
Regulations and treatment technologies are necessary to manage its environmental footprint.
Handling and Safety
Safe Handling of Cresol
Protective Measures and Equipment
To safely handle cresol, workers should use:
- Protective clothing
- Chemical-resistant gloves
- Eye protection
These precautions help prevent direct contact with the skin and eyes, reducing the risk of injury.
First Aid and Emergency Procedures
In case of exposure to cresol:
- Remove contaminated clothing.
- Rinse affected skin with plenty of water.
- Seek medical attention if necessary.
Prompt action can significantly reduce the severity of potential injuries.
Safe Handling of Phenol
Recommended Safety Practices
When dealing with phenol, it is crucial to:
- Ensure adequate ventilation.
- Use protective barriers.
- Implement strict containment measures.
These practices help maintain a safe working environment and prevent accidental exposure.
Emergency Response to Exposure
In an emergency involving phenol:
- Flush affected areas with water immediately.
- Remove any contaminated clothing carefully.
- Provide proper medical evaluation and support.
Quick and efficient emergency responses are vital to minimize health risks.
Regulatory Standards
Cresol Regulations
Global Safety Guidelines and Restrictions
Cresol is subject to stringent safety regulations globally, which dictate:
- Handling practices
- Exposure limits
- Disposal requirements
Adhering to these guidelines ensures that cresol use does not pose undue risks to health or the environment.
Industry-specific Standards
Different industries may have additional standards that apply specifically to their use of cresol, such as in pharmaceuticals and agriculture. These standards are designed to cater to the unique risks and usage patterns in each sector.
Phenol Regulations
Safety Protocols and Exposure Limits
Phenol’s handling and use are governed by comprehensive safety protocols which include:
- Maximum allowable exposure levels
- Mandatory safety training for handlers
- Specific guidelines for storage and transport
These regulations are crucial for ensuring safe use across various applications.
Compliance in Different Sectors
Compliance with phenol regulations is mandatory in sectors like plastics manufacturing, healthcare, and construction. Regular audits and inspections ensure that these standards are met, promoting safety and environmental sustainability.
Frequently Asked Questions
What is Cresol?
Cresol is a type of methylphenol with different isomers, including ortho-, meta-, and para-cresol. It is derived from coal tar or petroleum and is used primarily as a disinfectant, in pesticide formulations, and in the synthesis of other chemicals.
How is Phenol Produced?
Phenol is primarily produced through the cumene process, which involves the oxidation of cumene (isopropylbenzene) followed by its acid-catalyzed cleavage. This method is preferred for its efficiency and is widely used in industrial settings to meet large-scale demands.
What are the Main Uses of Phenol?
Phenol is extensively used in the manufacture of plastics, particularly in producing polycarbonate and epoxy resins. It also serves as a precursor to many pharmaceuticals and is an essential ingredient in the production of detergents and herbicides.
Are Cresol and Phenol Dangerous?
Both compounds can be toxic and pose health risks if not handled properly. Cresol can cause severe irritation and burns upon contact with skin, while phenol is toxic through inhalation, ingestion, or skin absorption, necessitating stringent safety measures during handling.
How do Environmental Regulations Affect Phenol and Cresol Usage?
Environmental regulations impact the use of both phenol and cresol by setting exposure limits and guidelines for safe disposal. These regulations are crucial to prevent environmental contamination and protect public health from the adverse effects of these chemicals.
Conclusion
Cresol and phenol are cornerstone chemicals with significant roles in many sectors. Understanding their differences is not just academic but a practical necessity for professionals in chemical manufacturing, healthcare, and environmental management. Their distinct properties and applications underscore the importance of specialized knowledge and regulatory compliance in handling these compounds.
This exploration of cresol and phenol highlights the depth and breadth of organic chemistry’s impact on modern industry and healthcare. As research progresses and industrial practices evolve, the roles of these essential chemicals will likely expand, bringing new challenges and opportunities for innovation.