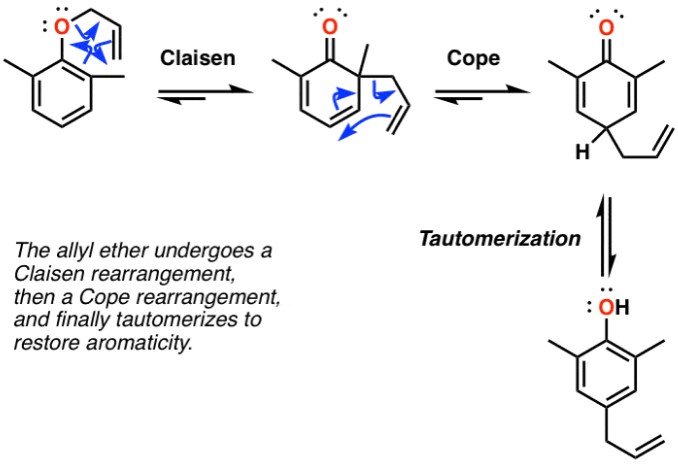
Organic chemistry, the study of carbon-containing compounds, is fundamental to understanding the chemistry of life and the synthesis of new materials. Among its many areas, rearrangement reactions stand out for their ability to transform molecules from one structure to another, enabling the creation of complex compounds from simpler ones. Two notable examples of such transformations are the Cope and Claisen rearrangements, each pivotal in synthetic organic chemistry for their unique outcomes and applications.
The Cope and Claisen rearrangements are thermal rearrangement reactions involving the migration of alkylidene or allyl groups in Cope and the migration of an allyl vinyl ether to form an ester or ketone in Claisen, respectively. These rearrangements are distinguished by their mechanisms, conditions, and the types of compounds they generate, making them indispensable tools in the chemist’s repertoire for constructing complex molecular architectures.
While the Cope rearrangement involves the movement of hydrogen and sigma bond migration within a molecule leading to the formation of different isomers under elevated temperatures, the Claisen rearrangement is characterized by its requirement for an ester or ketone outcome, often necessitating specific conditions such as the presence of an acid catalyst for certain substrates. Both reactions, however, are remarkable for their ability to profoundly change the structures and functional groups of molecules, showcasing the versatility and creativity inherent in organic synthesis.
Rearrangement Basics
Definition
Rearrangement reactions are chemical processes where the structure of a molecule changes as atoms within it move to different positions. This type of reaction is like rearranging the pieces of a puzzle to form a new picture. It’s fascinating because, unlike reactions that simply add or remove pieces (atoms or groups), rearrangement keeps the same pieces but changes their arrangement.
Importance
The role of rearrangement reactions in synthetic chemistry cannot be overstated. They are vital tools for creating complex molecules from simpler ones, often with high precision and efficiency. These reactions are the backbone of many synthetic strategies, leading to the discovery of new drugs, materials, and chemicals. Their importance lies in the ability to manipulate molecular structures, paving the way for advancements in various fields including pharmaceuticals, agriculture, and materials science.
Cope Rearrangement
Overview
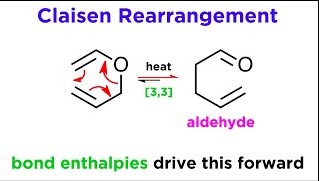
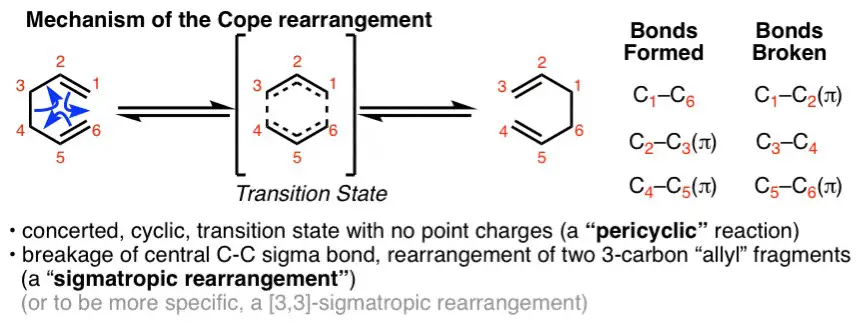
The Cope rearrangement is a thermal reaction involving the movement of hydrogen and sigma bonds within a molecule. This process is fascinating because it happens under the influence of heat alone, without the need for any external reagents or catalysts. It primarily affects molecules containing a 1,5-diene structure, leading to the formation of isomers – molecules with the same formula but a different arrangement of atoms.
Mechanism
The mechanism of the Cope rearrangement is a beautiful dance of electrons and atoms moving in a concerted manner:
- Initiation: The reaction starts with the heating of the substrate, energizing the molecule.
- Transition State Formation: The diene’s pi electrons shift, forming a six-membered ring transition state.
- Bond Rotation: Within this transition state, a bond rotation occurs, enabling the shift of the hydrogen and sigma bonds.
- Product Formation: The molecule then relaxes into its new structure, resulting in the rearrangement product.
This step-by-step process showcases the elegance of organic reactions, transforming molecules through a seamless flow of energy and movement.
Conditions
The success of the Cope rearrangement depends on several factors:
- Temperature: Generally requires high temperatures, often above 150°C.
- Solvents: Non-polar solvents are preferred as they facilitate the reaction by not interfering with the migrating groups.
Understanding these conditions is crucial for effectively employing this reaction in synthetic pathways.
Applications
The practical uses of the Cope rearrangement in organic synthesis are numerous. It serves as a powerful method for creating complex molecular structures, including natural products and polymers. Its ability to rearrange carbon skeletons makes it invaluable for building molecules with precise architectures, essential in drug development and materials science.
Claisen Rearrangement
Overview
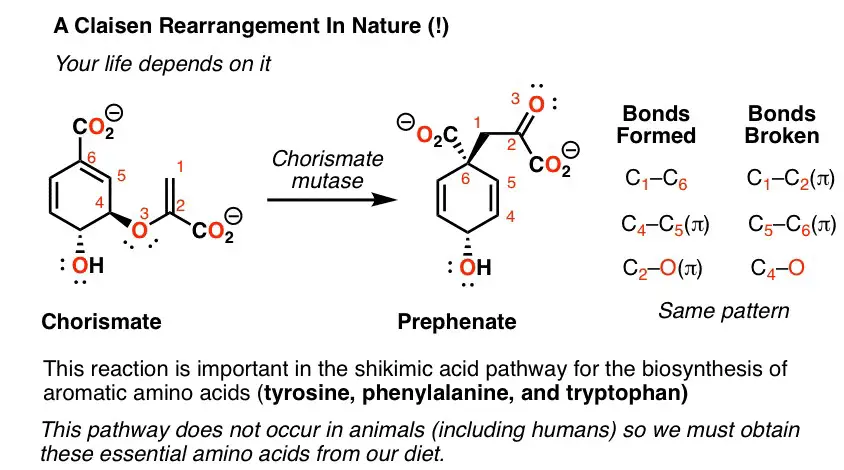
The Claisen rearrangement is another pivotal reaction in organic chemistry, involving the shift of an allyl vinyl ether to form an ester or ketone. Unlike the Cope rearrangement, the Claisen rearrangement can require specific conditions like the presence of an acid catalyst for certain substrates. It’s a versatile reaction, applicable to a wide range of substrates, leading to the formation of complex molecular structures.
Mechanism
The Claisen rearrangement mechanism unfolds through a series of well-coordinated steps:
- Initiation: Heating or the presence of a catalyst activates the substrate.
- Transition State Formation: The substrate forms a five-membered ring transition state, facilitated by the movement of electrons.
- Rearrangement: The allyl group shifts positions with the oxygen, forming a new carbon-oxygen bond.
- Product Formation: The final product, an ester or ketone, emerges as the molecule settles into its new configuration.
This mechanism illustrates the transformative power of rearrangement reactions, altering the connections between atoms to forge new molecules.
Conditions
Optimal conditions for the Claisen rearrangement include:
- Temperature: Typically requires moderate to high temperatures.
- Catalysts: Acid catalysts may be necessary for certain substrates.
- Solvents: The choice of solvent can vary based on the substrate but often involves polar solvents.
Tailoring these conditions to the specific needs of the reaction can greatly influence its outcome and yield.
Applications
The Claisen rearrangement has broad applications in the synthesis of complex molecules. It’s particularly useful in the formation of compounds with quaternary carbon centers, a challenge in organic synthesis. This rearrangement has found use in the production of pharmaceuticals, agrochemicals, and fragrances, demonstrating its versatility and importance in the chemical industry.
Key Differences
Reaction Conditions
Temperature and Solvent Comparison
The Cope and Claisen rearrangements differ significantly in their reaction conditions, particularly in terms of temperature and solvent requirements. The Cope rearrangement typically unfolds at high temperatures, often exceeding 150°C, and favors non-polar solvents that do not interfere with the migrating groups. On the other hand, the Claisen rearrangement can occur at lower temperatures, especially when acid catalysts are employed, and may utilize a broader range of solvents, including polar types, to facilitate the reaction.
Mechanism Contrast
Key Mechanistic Differences
At the heart of their distinction lies the mechanistic differences between the two rearrangements. The Cope rearrangement operates through a six-membered transition state, allowing for the movement of hydrogen and sigma bonds without the aid of external reagents. Conversely, the Claisen rearrangement involves a five-membered transition state with an allyl vinyl ether group migrating to form an ester or ketone, often requiring specific conditions or catalysts to proceed.
Outcome Diversity
Types of Products Formed
The types of products formed through these rearrangements underscore their differences. The Cope rearrangement yields isomers by rearranging existing bonds within molecules, primarily affecting compounds with a 1,5-diene structure. The Claisen rearrangement, however, results in the formation of esters or ketones from allyl vinyl ethers, showcasing its ability to create functionally diverse molecules.
Applications
Comparison in Synthetic Applications
When considering their applications in synthetic chemistry, both rearrangements offer valuable but distinct capabilities. The Cope rearrangement is particularly useful for creating complex isomers and has been applied in the synthesis of natural products and polymers. The Claisen rearrangement shines in constructing molecules with quaternary carbon centers, proving essential in pharmaceutical, agrochemical, and fragrance industries.
Similarities
Despite their differences, the Cope and Claisen rearrangements share important similarities. Both play crucial roles in organic synthesis, enabling chemists to manipulate molecular structures in sophisticated ways. Moreover, they both involve the movement of groups within molecules, although through different mechanisms and under varying conditions.
Role in Organic Synthesis
The shared role of these rearrangements in organic synthesis is to provide versatile pathways for constructing complex molecular architectures. They enable the transformation of simpler molecules into more complex ones, facilitating the development of new drugs and materials.
Types of Molecules Involved
Both rearrangements predominantly affect organic compounds containing multiple bonds or ether groups. While the specific structures involved differ, the underlying principle of rearranging existing atomic or group arrangements to forge new molecular configurations is a common thread.
Choosing Between Cope and Claisen
When faced with the decision of employing the Cope versus the Claisen rearrangement, several factors must be considered. These include the structure of the reactants, the desired product, and the specific conditions under which the reaction can successfully proceed.
Factors to Consider
Reactant Structure, Desired Product
The choice between these rearrangements hinges on the reactant structure and the desired product. For molecules with a 1,5-diene structure where isomerization is the goal, the Cope rearrangement is preferred. Conversely, for synthesizing esters or ketones from allyl vinyl ethers, the Claisen rearrangement is more appropriate.
Examples
Case Studies Showcasing Decision-Making Process
Case studies in synthetic chemistry highlight the decision-making process between these rearrangements. For instance, the synthesis of complex natural products might leverage the Cope rearrangement for its ability to create diverse isomers. Meanwhile, the synthesis of a pharmaceutical compound requiring a quaternary carbon center might benefit from the specific capabilities of the Claisen rearrangement.
Recent Advances
The field of synthetic chemistry continuously evolves, with recent advances in both the Cope and Claisen rearrangements offering new possibilities.
Innovations in Reaction Conditions
New Applications in Synthesis
Innovations have led to more efficient and selective reaction conditions, broadening the applicability of these rearrangements. For example, modified catalysts and solvent systems have enabled the Claisen rearrangement to proceed at lower temperatures or with greater selectivity. Similarly, the discovery of photochemical methods to initiate the Cope rearrangement has opened up new avenues for conducting these reactions under milder conditions, expanding their utility in the synthesis of sensitive compounds.
Frequently Asked Questions
What is a rearrangement reaction?
Rearrangement reactions involve the structural reorganization of molecules, where atoms and groups of atoms shift positions, resulting in a different compound. These transformations are critical in organic chemistry for the synthesis of complex molecules from simpler precursors, offering pathways to diversity in molecular design and functionality.
How does the Cope rearrangement differ from the Claisen rearrangement?
The Cope rearrangement is a thermal process that involves the migration of alkylidene or allyl groups within a molecule, typically requiring only heat. In contrast, the Claisen rearrangement involves the migration of an allyl vinyl ether to form an ester or ketone, often under specific conditions such as the presence of an acid catalyst. The key difference lies in their mechanisms, the conditions under which they occur, and the types of products they yield.
Why are the Cope and Claisen rearrangements important in synthetic chemistry?
The Cope and Claisen rearrangements are pivotal in synthetic chemistry for their ability to construct complex molecular structures from simpler ones. They enable chemists to synthesize a wide variety of compounds, ranging from pharmaceuticals to materials science applications, showcasing the versatility and ingenuity of organic synthesis.
Conclusion
The exploration of Cope and Claisen rearrangements offers a window into the sophisticated world of organic synthesis, where the rearrangement of molecules serves as a fundamental tool for constructing complex chemical structures. These reactions not only exemplify the creativity and strategic thinking required in organic chemistry but also underscore the discipline’s central role in advancing science and technology through the creation of novel compounds.
Understanding the nuances between the Cope and Claisen rearrangements, from their mechanisms to their applications, is crucial for chemists aiming to harness these reactions for innovative syntheses. As the field of organic chemistry continues to evolve, the exploration and application of such rearrangements will undoubtedly play a pivotal role in the discovery and development of new materials and therapeutics, highlighting the endless possibilities that arise from the art and science of chemical transformation.